Acute ST-segment elevation myocardial infarction patients presenting persistent no-flow after wire insertion have a lower survival rate despite successful mechanical intervention. The neutrophil-to-lymphocyte ratio has been associated with increased mortality and worse clinical outcomes in ST-segment elevation myocardial infarction. We hypothesized that an elevated neutrophil-to-lymphocyte ratio would also be associated with a persistent Thrombolysis In Myocardial Infarction flow grade of 0 after wire insertion in patients undergoing primary percutaneous coronary intervention.
METHODS:A total of 644 patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention within 12 hours of symptom onset were included in our study. Blood samples were drawn immediately upon hospital admission. The patients were divided into 3 groups according to their Thrombolysis In Myocardial Infarction flow grade: Thrombolysis In Myocardial Infarction flow grade 0 after wire insertion, Thrombolysis In Myocardial Infarction flow grade 1-3 after wire insertion and Thrombolysis In Myocardial Infarction flow grade 1-3 at baseline.
RESULTS:The neutrophil-to-lymphocyte ratio was significantly higher in the group with Thrombolysis In Myocardial Infarction flow grade 0 after wire insertion compared with the group with Thrombolysis In Myocardial Infarction flow grade 1-3 after wire insertion and the group with Thrombolysis In Myocardial Infarction flow grade 1-3 at baseline. The group with Thrombolysis In Myocardial Infarction flow grade 0 after wire insertion also had a significantly higher in-hospital mortality rate. Persistent coronary no-flow after wire insertion was independently associated with the neutrophil-to-lymphocyte ratio.
CONCLUSIONS:An increased neutrophil-to-lymphocyte ratio on admission is significantly associated with persistent coronary no-flow after wire insertion in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention.
Primary percutaneous coronary intervention (PPCI) is an established therapeutic strategy for ST-elevation myocardial infarction (STEMI) 1, and the Thrombolysis In Myocardial Infarction (TIMI) flow grade is a scoring method for assessing coronary blood flow both before and after reperfusion 2. The baseline TIMI flow before intervention in an infarct-related artery (IRA) has been previously shown to influence mortality in patients with STEMI undergoing PPCI 3. However, the patency of the IRA before PPCI in patients with STEMI is a major determinant of TIMI flow 3 after PPCI, which is associated with an improved clinical outcome 4. In contrast, STEMI patients displaying persistent no-flow after wire insertion (AWI) have a lower survival rate despite apparently successful PPCI 5.
Atherosclerosis is an inflammatory disease 6. Inflammation promotes coronary atherosclerotic plaque rupture and atherothrombosis 7, which are the main mechanisms in the pathophysiology of acute STEMI. Leukocytosis is a marker of inflammation and is associated with the inflammatory response at plaque sites in patients with STEMI 6-8. As part of this inflammatory reaction, cytokines such as interleukin (IL)-6, IL-8 and CD40 ligand trigger the upregulation of monocyte tissue factor expression, which may facilitate the extrinsic pathway of the coagulation cascade 9. The neutrophil-to-lymphocyte ratio (NLR) has also emerged as an important inflammatory marker for cardiovascular risk stratification 10. Previously, several studies established that an elevated NLR was associated with early IRA patency before PPCI, the development of no-reflow after PPCI, increased mortality and worse cardiovascular outcomes in acute STEMI 11-13. However, the relationship between the admission NLR and coronary flow AWI in STEMI patients has not been assessed. Thus, we investigated whether an elevated NLR, as measured on admission, is associated with a persistent TIMI flow grade of 0 AWI in patients with acute STEMI undergoing mechanical reperfusion.
METHODSBetween July 2012 and April 2014, consecutive patients who were hospitalized at our institution because of acute STEMI and who underwent PPCI within 12 hours after diagnosis were enrolled in our study. Initially, 669 patients were eligible for this study. In total, 25 patients were excluded from the study for the following reasons: 5 patients had unavailable laboratory data, 2 patients had multivessel intervention, 10 patients had been treated with an urgent coronary artery bypass graft due to failed PPCI or had coronary anatomy that was not amenable to PPCI, 3 patients had severe renal failure and 5 patients had acute or chronic infection/inflammation. Therefore, a total of 644 patients were ultimately included in our study. The mean age was 60±13 years and 480 (74.5%) of the patients were men.
The definition of STEMI was based on criteria for the classic symptoms of coronary ischemia and the detection of a 1-mm ST-segment elevation in the inferior leads, a 2-mm ST-segment elevation in the anterior chest leads occurring in two contiguous leads (or reciprocal ST depression ≥1 mm in V1 or V2), or the presence of a new (or presumably new) left bundle branch block. Patients with active infection, previously proven chronic inflammatory disease, known malignancy, advanced-stage liver or renal disorders, or fibrinolytic administration in the previous 30 days were excluded from this study. To avoid the confounding effect of multiple lesions, only patients who underwent PPCI in a single IRA, without the treatment of additional lesions were considered. The patients were divided into 3 groups according to their TIMI flow grade: AWI TIMI flow grade 0 group, AWI TIMI flow grade 1-3 group and baseline TIMI flow grade 1-3 group. The study protocol was approved by the institutional review board at our hospital and all patients gave written informed consent before study entry.
All procedural parameters were evaluated by two independent experienced interventional cardiologists using quantitative cardiovascular angiographic software (Axiom Sensis XP; Siemens, Munich, Germany). The Syntax scores of all patients (except for coronary artery bypass grafting (CABG) patients) were calculated by two independent experienced interventional cardiologists who were blinded to the identities of the patients and to the patients' clinical information from baseline diagnostic angiography 14. Each lesion with ≥50% diameter stenosis in vessels ≥1.5 mm in diameter was scored using an online calculator, version 2.1, at www.syntaxscore.com. Additionally, the TIMI flow grade was visually assessed in all patients. The TIMI flow grade was measured as follows: 0 = complete vessel occlusion with no angiographic visualization of the vessel beyond the site of stenosis (no perfusion); 1 = penetration without perfusion; 2 = partial reperfusion and 3 = complete filling of the distal vessel by the third cardiac cycle (complete reperfusion) 15. As part of the protocol in this study, operators were requested to film coronary flow before (pre-PPCI flow) and immediately after (TIMI AWI) wire insertion, which was defined as satisfactory positioning of the wire completely down the length of the IRA, as well as upon removal of the wire after the intervention (post-PPCI flow). Procedural success for coronary stent placement was defined as achieving a minimum stenosis diameter reduction to less than 20% in the IRA, along with TIMI grade 3 coronary flow. The median door-to-balloon interval was 40 (32-55) minutes in our study.
Blood samples were drawn immediately upon hospital admission, before PPCI. Routine complete blood cell counts (XE-2100; Sysmex Inc., Japan) and blood chemistry measurements (glucose, creatinine) were then performed at our hospital. Cardiac enzyme (creatinine kinase-myocardial band (CK-MB), troponin T) levels, lipid profiles and high-sensitivity C-reactive protein (hs-CRP) levels were also measured in all patients. The NLR was calculated as the ratio of the neutrophil count to the lymphocyte count. Moreover, to evaluate kidney function, the estimated glomerular filtration rate (eGFR) was obtained by applying the Modification of Diet in Renal Disease Study formula. To assess cardiac function, transthoracic echocardiography was routinely performed on each patient within 48 hours following PPCI (Vivid 3; GE Medical Systems, Horten, Norway). The left ventricular ejection fraction (LVEF) was also measured using the Simpson method according to the recommendations of the American Society of Echocardiography 16.
All patients received a single loading dose of 600 mg clopidogrel and 300 mg aspirin immediately after arrival at the hospital. In addition, all patients received an intravenous bolus injection of 5000 U heparin before angiography. Baseline coronary angiography was performed by the femoral approach using the standard Judkins technique (Siemens Axiom Artis zee 2011; Siemens Healthcare, Erlangen, Germany). According to established guidelines, only culprit lesions were treated using standard PCI techniques and a 6-Fr guiding catheter (Launcher; Medtronic, Minneapolis, Minnesota, USA). According to patient characteristics and angiographic features, the selection of the type of stent (bare metal or drug eluting) and the decision to use a glycoprotein IIb/IIIa antagonist (tirofiban) or an intra-aortic balloon pump were left to the discretion of the operator. Additionally, the use of pre- or post-dilation and thrombus aspiration was left to the discretion of the treating physician. The IRA was the only target of the procedure, except in the case of cardiogenic shock. A 75 mg clopidogrel dose was administered for at least 12 months after the PPCI and 100 mg aspirin was prescribed indefinitely.
Statistical analysis was performed using the SPSS 18.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA). Quantitative variables are expressed as the mean±standard deviation or as the median and interquartile range. Continuous variables were analyzed for normal distribution using the Kolmogorov-Smirnov test and analyzed for homogeneity using the Levene test. Comparisons of parametric values among groups were performed using one-way ANOVA. Comparisons of non-parametric values among groups were performed using the Kruskal-Wallis test. Tukey's HSD (for parametric variables) and the Bonferroni-adjusted Mann-Whitney U-test (for non-parametric variables) were used as post hoc tests for multiple comparisons between groups. A two-tailed p-value<0.05 was considered statistically significant. A receiver operating characteristic (ROC) analysis was also performed to identify the best cutoff value for the NLR and the sensitivity and specificity at that point were obtained for predicting persistent coronary no-flow AWI. Univariate logistic regression was additionally used to identify independent predictors of TIMI flow grade 0 AWI. After performing the univariate analysis, significant variables (age, smoking, systolic blood pressure (SBP), LVEF, hemoglobin, eGFR, total cholesterol, NLR, peak CK-MB, hs-CRP, white blood cell (WBC) count, Syntax score and Killip class ≥2 on admission) were used in a multivariate logistic regression analysis. We also performed a multivariate logistic regression analysis to evaluate data the NLR versus in-hospital mortality, coronary no-flow, peak CK-MB and the Syntax score and in-hospital mortality versus the NLR cutoff value, the Syntax score, peak CK-MB and no-flow in our study population.
RESULTSThe baseline clinical characteristics of the patients in the 3 groups, divided according to the coronary TIMI flow grade are shown in Table1. The group with persistent coronary no-flow AWI was older, contained fewer smokers, had a lower initial SBP and lower LVEF values, and more commonly had Killip class ≥2 on admission. Compared with patients with TIMI flow grade 1-3 at baseline and TIMI flow grade 1-3 AWI, patients with persistent coronary no-flow (TIMI 0) AWI had higher cardiac enzyme (CK-MB, troponin T) and hs-CRP levels, higher WBC and neutrophil counts and a lower lymphocyte count. These patients also had significantly lower hemoglobin, total cholesterol and triglyceride levels (Table2). The NLR was significantly higher in the AWI TIMI flow 0 group compared with the AWI TIMI flow 1-3 and baseline TIMI flow 1-3 groups (7.74±4.96, 4.22±2.53 and 3.39±2.03, respectively, p<0.001). Figure1 and Table2 show the frequency distribution of the NLR according to the groups.
Baseline characteristics and prior medications of the study population.
| Baseline TIMI Flow Grade 0 (n = 324) | ||||
|---|---|---|---|---|
| After Wire Insertion | ||||
| Variable | TIMI 0 (n = 185) | TIMI 1-3 (n = 139) | Baseline TIMI Flow Grade 1-3 (n = 320) | p value |
| Age, years | 63±13 | 59±13 | 59±12 | 0.001 |
| Male gender, n (%) | 129 (69.7) | 105 (75.5) | 246 (76.9) | 0.197 |
| Body mass index, kg/m2 | 27.2±4.4 | 27.3±4.0 | 27.9±4.1 | 0.237 |
| Hypertension, n (%) | 83 (44.9) | 53 (38.1) | 123 (38.4) | 0.311 |
| Diabetes mellitus, n (%) | 65 (35.1) | 34 (24.5) | 101 (31.6) | 0.116 |
| Smoking, n (%) | 80 (43.2) | 81 (58.3) | 159 (49.7) | 0.028 |
| Hyperlipidemia, n (%) | 56 (30.2) | 50 (36.0) | 117 (36.6) | 0.293 |
| Family history, n (%) | 43 (23.2) | 44 (31.7) | 86 (26.9) | 0.240 |
| Prior stroke, n (%) | 6 (3.2) | 0 (0) | 6 (1.9) | 0.102 |
| Prior CABG, n (%) | 4 (2.2) | 4 (2.9) | 11 (3.4) | 0.716 |
| Prior myocardial infarction, n (%) | 11 (5.9) | 3 (2.2) | 13 (4.1) | 0.239 |
| Systolic blood pressure, mmHg | 122±25 | 127±26 | 130±23 | 0.006 |
| Heart rate, beats/min | 81±17 | 78±15 | 79±15 | 0.185 |
| Anterior infarct, n (%) | 86 (46.5) | 48 (34.5) | 151 (47.2) | 0.019 |
| Killip class ≥2 on admission | 29 (15.7) | 7 (5.0) | 14 (4.4) | <0.001 |
| LVEF, % | 42±10 | 45±9 | 47±10 | <0.001 |
| Symptoms prior to PPCI, min | 222±115 | 210±136 | 175±121 | 0.225 |
| <6 hours, n (%) | 134 (72.4) | 113 (81.3) | 241 (75.3) | 0.176 |
| ≥6 hours, n (%) | 51 (27.6) | 26 (18.7) | 79 (24.7) | |
| Preinfarct angina, n (%) | 46 (25.0) | 41 (29.7) | 111 (34.7) | 0.557 |
| Medications before hospitalization (%) | ||||
| Aspirin | 18.5 | 20.8 | 23.8 | 0.190 |
| Clopidogrel | 9.3 | 12.5 | 14.2 | 0.458 |
| ACEI or ARB | 32.0 | 28.3 | 33.3 | 0.708 |
| Beta-blocker | 12.0 | 8.9 | 7.8 | 0.707 |
| Statin | 4.0 | 4.4 | 4.4 | 0.991 |
TIMI: Thrombolysis In Myocardial Infarction; CABG: coronary artery bypass grafting; LVEF: left ventricular ejection fraction; PPCI: primary percutaneous coronary intervention; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Baseline biochemical and hematologic measurements for the study population.
| Baseline TIMI Flow Grade 0 (n = 324) | ||||
|---|---|---|---|---|
| After Wire Insertion | ||||
| Variable | TIMI 0 (n = 185) | TIMI 1-3 (n = 139) | Baseline TIMI Flow Grade 1-3 (n = 320) | p value |
| Serum glucose (mg/dL) | 152±71 | 140±74 | 143±69 | 0.260 |
| Hemoglobin A1c (%) | 6.71±1.75 | 6.62±1.64 | 6.85±2.03 | 0.511 |
| Creatinine (mg/dL) | 1.15±0.34 | 1.09±0.50 | 1.09±0.26 | 0.157 |
| eGFR (mL/min/1.73 m2) | 67±20 | 76±20 | 73±19 | 0.001 |
| Peak CK-MB (ng/mL) | 137 (60-295) | 116 (63-181) | 59 (32-127) | <0.001 |
| Peak troponin T (ng/mL) | 3245 (1279-6648) | 1850 (838-4633) | 1030 (431-2214) | <0.001 |
| hs-CRP (mg/dL) | 9.03±3.21 | 7.98±3.63 | 6.95±3.84 | <0.001 |
| Low-density lipoprotein (mg/dL) | 112±40 | 124±38 | 126±41 | 0.001 |
| High-density lipoprotein (mg/dL) | 40.4±10.2 | 40.5±9.0 | 40.5±9.4 | 0.995 |
| Triglyceride (mg/dL) | 122 (82-171) | 137 (106-201) | 149 (104-198) | 0.010 |
| Total cholesterol (mg/dL) | 180±47 | 194±42 | 192±49 | <0.001 |
| Hemoglobin (g/L) | 13.73±2.04 | 14.26±1.78 | 14.29±1.76 | 0.003 |
| Platelet count (x103/µL) | 243±76 | 239±62 | 244±70 | 0.791 |
| Mean platelet volume (fL) | 8.84±1.18 | 8.63±0.90 | 8.71±1.03 | 0.121 |
| White blood cell count (x103/µL) | 12.44±3.70 | 11.86±3.78 | 11.33±3.39 | 0.001 |
| Neutrophil count | 8.42±3.26 | 8.81±3.31 | 7.26±2.81 | <0.001 |
| Lymphocyte count | 1.62±0.75 | 2.41±1.08 | 2.69±1.38 | <0.001 |
| Neutrophil-to-lymphocyte ratio | 7.74±4.96 | 4.22±2.53 | 3.39±2.03 | <0.001 |
| Complete STR on electrocardiography | 83 (44.8) | 102 (73.7) | 247 (77.2) | <0.001 |
| In-hospital mortality | 13 (7.0) | 6 (4.3) | 7 (2.2) | 0.028 |
TIMI: Thrombolysis In Myocardial Infarction; eGFR: estimated glomerular filtration rate; CK-MB: creatine kinase-myocardial band; hs-CRP: high-sensitivity C-reactive protein; STR: ST-segment resolution.
A total of 26 (4.0%) cases of mortality were documented during hospitalization. The AWI TIMI flow 0 group had a higher rate of in-hospital mortality compared with the AWI TIMI flow 1-3 and baseline TIMI flow 1-3 groups (13 (7.0), 6 (4.3) and 7 (2.2), respectively, p = 0.028) (Table2). We found that in-hospital mortality was independently associated with an increased NLR and increased no-flow (OR 1.52, p = 0.004 and OR 3.1, p<0.001, respectively). In addition, the percentage of complete (≥70%) ST-segment resolution on electrocardiography after PPCI was significantly lower in the AWI TIMI flow 0 group compared with the AWI TIMI flow 1-3 and baseline TIMI flow 1-3 groups (p<0.001).
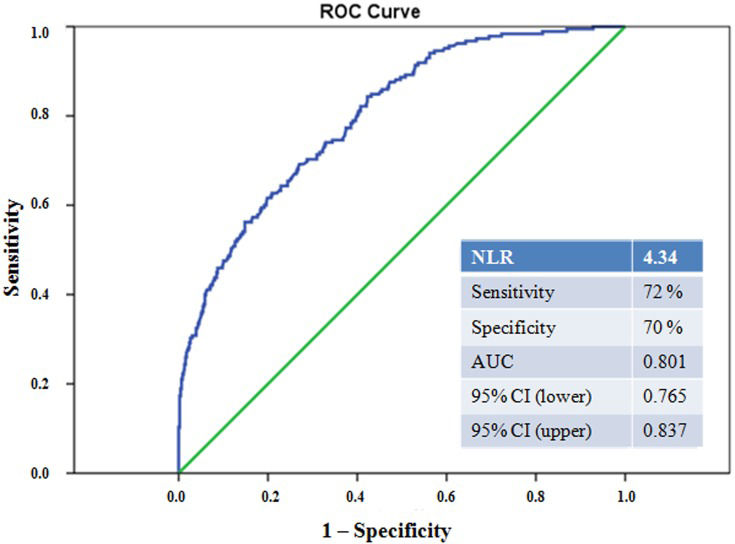
The baseline angiographic and procedural characteristics of the study population are presented in Table3. The number of diseased vessels and the prevalence of multivessel disease were similar among the groups (p = 0.252 and p = 0.277, respectively). The IFAs of patients with TIMI flow grade 1-3 AWI were less frequently located in the left anterior descending coronary artery, whereas in patients with baseline TIMI flow grade 0, the culprit lesions were more commonly located in the right coronary artery (p = 0.024). TIMI flow grade 0 was present before PPCI in 51.9% (n = 334) of infarct vessels and suboptimal flow (TIMI flow grade 1-2) was found in 25.0% (n = 161), whereas the remainder of infarct arteries presented with TIMI flow grade 3. As shown in Table3, a final TIMI flow grade of 3 was achieved in 56.2% (n = 104) of patients with TIMI flow grade 0 AWI and in 87.8% (n = 122) of patients with TIMI flow grade 1-3 AWI; the remainder of patients had TIMI flow grade 1-3 at baseline. However, patients with TIMI flow grade 0 with persistent coronary no-flow after passage of the wire had a significantly lower prevalence of stent implantation, a lower rate of direct stenting, a higher total stent length and higher rate of balloon predilatation compared with those with TIMI flow grade 1-3 AWI and TIMI flow grade 1-3 at baseline (p<0.001 in post hoc analysis) (Table3). Additionally, patients presenting with TIMI flow grade 0 had a significantly higher prevalence of tirofiban use compared with those with TIMI flow grade 1-3 at baseline (p = 0.006 in post hoc analysis). The multivariate logistic regression analysis showed that persistent coronary no-flow (TIMI 0) AWI was independently associated with the NLR (OR 1.421, 95% CI 1.274-1.585, p<0.001), peak CK-MB (OR 1.004, 95% CI 1001-1.007, p = 0.005) and the Syntax score (OR 1.058, 95% CI 1.021-1.096, p = 0.002) (Table4). In the ROC curve analysis, an NLR>4.34 independently predicted TIMI flow 0 AWI, with 72% sensitivity and 70% specificity (AUC 0.801, 95% CI 0.765-0.837) (Figure2). We also found that an NLR>4.34 was independently associated with the Syntax score (OR 1.035, p = 0.004), no-flow (OR 3.044, p<0.001) and peak CK-MB (OR 1.003, p = 0.001).
Baseline angiographic and procedural characteristics of the study population.
| Baseline TIMI Flow Grade 0 (n = 324) | ||||
|---|---|---|---|---|
| After Wire Insertion | ||||
| Variable | TIMI 0 (n = 185) | TIMI 1-3 (n = 139) | Baseline TIMI Flow Grade 1-3 (n = 320) | p value |
| Multivessel disease, n (%) | 105 (56.8) | 73 (52.5) | 158 (49.4) | 0.277 |
| Single-vessel disease, n (%) | 81 (43.8) | 66 (47.5) | 165 (51.6) | 0.550 |
| Double-vessel disease, n (%) | 62 (33.5) | 43 (30.9) | 95 (29.7) | |
| Triple-vessel disease, n (%) | 42 (22.7) | 30 (21.6) | 60 (18.8) | |
| Number of diseased vessels, n (%) | 1.79±0.79 | 1.74±0.79 | 1.72±0.78 | 0.252 |
| Infarct-related artery, n (%) | ||||
| Left anterior descending | 86 (46.5) | 49 (35.3) | 160 (50.0) | 0.024 |
| Left circumflex | 24 (13.0) | 20 (14.4) | 57 (17.8) | |
| Right | 75 (40.5) | 69 (49.6) | 101 (31.6) | |
| Left main | 0 (0) | 1 (0.7) | 2 (0.6) | |
| Chronic total occlusion, n (%) | 32 (16.3) | 16 (11.5) | 45 (14.1) | 0.329 |
| Thrombus aspiration, n (%) | 30 (16.2) | 21 (15.0) | 44 (13.8) | 0.560 |
| Stent deployed, n (%) | 155 (83.8) | 136 (97.8) | 302 (94.4) | <0.001 |
| Number of stents used | 1.32±0.60 | 1.26±0.54 | 1.23±0.45 | 0.232 |
| Total stent length, mm | 27.42±11.84 | 23.86±11.87 | 20.91±8.52 | <0.001 |
| Direct stenting, n (%) | 5 (2.7) | 78 (56.1) | 229 (71.6) | <0.001 |
| Stent with balloon predilatation, n (%) | 147 (79.5) | 56 (40.3) | 68 (21.3) | <0.001 |
| Tirofiban therapy, n (%) | 91 (49.2) | 68 (48.9) | 117 (36.6) | 0.006 |
| Procedural success, n (%) | 104 (56.2) | 122 (87.8) | 275 (89.6) | <0.001 |
| Syntax score | 20.4±8.3 | 18.4±8.0 | 12.7±7.8 | <0.001 |
Univariate and multivariate predictors of Thrombolysis In Myocardial Infarction 0 flow grade in ST-segment elevation myocardial infarction after wire insertion.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variable | Odds Ratio, 95% CI | p-value | Odds Ratio, 95% CI | p-value |
| Age | 1.026 (1.012-1.039) | <0.001 | 1.003 (0.973-1.033) | 0.864 |
| Smoking | 0.695 (0.493-0.980) | 0.038 | 0.657 (0.331-1.307 | 0.231 |
| Systolic blood pressure | 0.989 (0.982-0.996) | 0.002 | 1.004 (0.992-1.015) | 0.544 |
| Left ventricular ejection fraction | 0.950 (0.932-0.968) | <0.001 | 0.991 (0.960-1.024) | 0.591 |
| White blood cell count | 1.079 (1.030-1.130) | 0.001 | 0.929 (0.846-1.019) | 0.119 |
| Hemoglobin | 0.856 (0.782-0.937) | 0.001 | 0.906 (0.763-1.075) | 0.906 |
| Estimated glomerular filtration rate | 0.984 (0.975-0.992) | <0.001 | 0.994 (0.978-1.010) | 0.463 |
| Syntax score | 1.083 (1.060-1.106) | <0.001 | 1.058 (1.021-1.096) | 0.002 |
| Total cholesterol | 0.992 (0.988-0.996) | <0.001 | 0.994 (0.988-1.000) | 0.059 |
| Neutrophil-to-lymphocyte ratio | 1.466 (1.363-1.577) | <0.001 | 1.421 (1.274-1.585) | <0.001 |
| Creatine kinase-myocardial band | 1.006 (1.004-1.008) | <0.001 | 1.004 (1001-1.007) | 0.005 |
| High-sensitivity C-reactive protein | 1.152 (1.089-1.219) | <0.001 | 1.050 (0.973-1.133) | 0.212 |
| Killip class ≥2 on admission | 3.875 (2.145-6.993) | <0.001 | 1.581 (0.462-5.414) | 0.465 |
AWI: after wire insertion; TIMI: Thrombolysis In Myocardial Infarction; STEMI: ST-segment elevation myocardial infarction.
To the best of our knowledge, this is the first study to identify an association between the NLR and coronary flow AWI in patients with STEMI undergoing PPCI. The main finding in this study was that an increased NLR value on admission was a strong and independent predictor of persistent coronary no-flow AWI in patients with STEMI undergoing mechanical reperfusion. In addition, the patients with TIMI flow grade 0 AWI had a higher rate of in-hospital mortality.
The early restoration of coronary flow in an IRA recovers ventricular performance and decreases mortality in patients with STEMI 3. Brodie et al. 3 were the first to report a different survival pattern in patients presenting with TIMI flow grade 2 or 3 before intervention compared with patients with baseline TIMI flow grade 0 or 1. Moreover, STEMI patients presenting persistent no-flow AWI have a lower survival rate despite apparently successful mechanical intervention 5. Atherosclerosis is an inflammatory process 6 and inflammatory markers have been identified as useful predictors of clinical outcomes. In particular, the NLR has emerged as an important inflammatory marker for cardiovascular risk stratification 10. Akpek et al. 13 showed that the NLR was independently associated with the development of no-reflow and in-hospital major adverse cardiac events in patients with STEMI undergoing PPCI. In a recently published study, we also demonstrated that the NLR is associated with early patency of the IRA before PPCI in patients with STEMI 11.
There are several possible explanations for why an increased NLR value on admission was an independent predictor of persistent coronary no-flow AWI in patients with STEMI undergoing PPCI in our study.
First, the NLR is a sign of balance between neutrophil and lymphocyte counts in the body and is an indicator of systemic inflammation 17. Increased inflammation in patients with coronary artery disease may be linked to more extensive atheroma 18,19 and the association between thrombotic and inflammatory pathways in acute coronary syndromes has been previously shown. Atherosclerotic plaque rupture is an inflammatory process mediated by the complex interplay between innate neutrophil-mediated reactive immune responses and subsequent lymphocyte-mediated adaptive immune responses. Neutrophils are the first leukocytes found in the damaged myocardial area. Procoagulants are secreted locally by the leukocytes, increasing the oxidative and proteolytic damage. Lymphocytes also have an essential role in modulating the inflammatory response at different stages of the atherosclerotic process 20. In an acute setting, lymphopenia is a common finding during a stress response, secondary to increased levels of corticosteroids 21. Under pathologic conditions, defective clearance of apoptotic cells due to poor phagocytosis of apoptotic cells results in secondary, necrosis-inducing secretion of proinflammatory cytokines (tumor necrosis factor-α and IL-6). In addition, lymphopenia has been reported in critical inflammatory states due to increased lymphocyte apoptosis 22.
Second, neutrophil infiltration can contribute to no-reflow by increasing blood viscosity and hypercoagulability. As part of this inflammatory reaction, cytokines such as IL-6, IL-8 and CD40 ligand trigger the upregulation of monocyte tissue factor expression, which may facilitate the extrinsic pathway of the coagulation cascade 23. Additionally, the distal embolization of leukocytes and platelet-leukocyte aggregates might contribute to reduced downstream microvascular perfusion as well as to thrombosis and widespread myocardial inflammation 8.
Third, it has been shown that a higher WBC count is correlated with the infarct size 24. After AMI, the release of chemoattractants draws neutrophils into the infarct zone during the first 6 hours of myocardial reperfusion; during the next 24 hours, the cells migrate into the myocardial tissue 24. Neutrophil infiltration is regulated through a complex sequence of molecular steps involving selectins and integrins, which mediate leukocyte rolling and adhesion to the endothelium 25. These neutrophils cause proteolytic and oxidative damage to the endothelial cells, plug the microvasculature, and induce hypercoagulability and may promote infarct expansion 24,25. As we have found that peak CK-MB and troponin T levels were elevated and the LVEF was decreased in patients with TIMI flow grade 0 AWI, the NLR may act as a combined surrogate marker for both the reactive and the adaptive components of the inflammatory response that results in plaque rupture, ischemic myocardial damage, adverse ventricular remodeling and consequent LV dysfunction 26.
Our study has certain limitations. First, inflammatory markers other than hs-CRP were not analyzed and compared with the NLR. Second, the myocardial blush score, which is a well-known prognostic indicator in STEMI patients 27, was not assessed in our study. Finally, the NLR was measured only at the time of admission to evaluate its prognostic impact. In conclusion, the NLR is a strong independent predictor of persistent coronary no-flow AWI in patients with STEMI undergoing mechanical revascularization. Patients with STEMI in whom persistent no-flow AWI is detected during PPCI are at greater risk for in-hospital mortality. As an early and readily available prognostic marker, the NLR may thus be useful in the early risk stratification of STEMI patients treated via PPCI.
AUTHOR CONTRIBUTIONSKurtul A, Murat SN and Yarlioglues M wrote the manuscript, performed the statistical analyses and were responsible for the final editing. Duran M, Celik IE, Kilic A and Ocek AH collected the data.
No potential conflict of interest was reported.