An elevated red cell distribution width has been recognized as a predictor of various cardiovascular diseases. Slow coronary flow syndrome is an important angiographic clinical entity with an unknown etiology. This study aimed to examine the relationship between red cell distribution width and the presence of slow coronary flow syndrome.
METHODSIn total, 185 patients with slow coronary flow syndrome and 183 age- and gender-matched subjects with normal coronary flow (controls) were prospectively enrolled in this study. Red cell distribution width and C-reactive protein were measured upon admission, and the results were compared between the patients with slow coronary flow syndrome and normal controls.
RESULTSRed cell distribution width levels were significantly higher in the patients with slow coronary flow syndrome than the normal controls. Moreover, the data showed that the plasma C-reactive protein levels were also higher in the patients with slow coronary flow syndrome than in the normal controls. In addition, a multivariate analysis indicated that C-reactive protein and red cell distribution width were the independent variables most strongly associated with slow coronary flow syndrome. Finally, the red cell distribution width was positively correlated with C-reactive protein and mean thrombosis in the myocardial infarction frame counts of the patients with slow coronary flow syndrome.
CONCLUSIONThe data demonstrated that red cell distribution width levels are significantly higher and strongly positively correlated with both C-reactive protein and thrombosis in the myocardial infarction frame counts of patients with slow coronary flow syndrome. These findings suggest that red cell distribution width may be a useful marker for patients with slow coronary flow syndrome.
Slow coronary flow syndrome (SCFS) is an angiographic observation characterized by angiographically normal or near-normal coronary arteries with the delayed progression of contrast dye injected into the coronary tree (1–3). Despite the relatively good prognosis of SCFS patients, the chronic nature of the persistent chest discomfort can significantly impair quality of life (1). In addition, many SCFS patients suffer from recurrent chest pain, resulting in emergency room evaluations, hospitalizations, and repeat cardiac catheterizations, according to a previous study (2). More importantly, life threatening arrhythmias and sudden cardiac death have also been reported in these patients (3). Although intensive studies have been performed over the past several decades, the underlying mechanism responsible for SCFS remains unknown (1–3).
Red cell distribution width (RDW), a part of a routine complete blood count, measures the variability in the size of circulating erythrocytes, which has been utilized in the differential diagnosis of anemia (4,5). Recently, elevated RDW levels have been suggested as a readily available marker for and independent predictor of various cardiovascular diseases, including acute and chronic arterial diseases (6–10), percutaneous coronary intervention after acute myocardial infarction (11,12), and heart failure (13,14) in the elderly and general populations (15,16).
It has been reported that inflammation may be a potential mechanism underlying elevated RDW levels in patients with chronic and acute cardiovascular diseases (6–14). Additionally, emerging data have suggested that inflammation may play a role in the pathogenesis of SCFS (3). Accordingly, we hypothesized that an elevated RDW level might be significantly associated with the presence of SCFS because of the inflammatory features of this unique disorder. Therefore, in the present study, we prospectively examined the relationship between RDW levels and the presence of SCFS.
METHODSSubjectsThis observational study was derived from a cohort of patients prospectively entered into a database. The purpose was to assess the prognostic significance of various plasma biomarkers in patients with known or suspected coronary artery disease in our divisions at the Fu Wai and Anzhen Hospitals Beijing. The study was approved by the local ethics committee and complied with the Declaration of Helsinki. The study population was selected between December 2009 and March 2012 based on the presence of angina-like chest pain or a positive treadmill exercise test. Finally, 185 consecutive SCFS patients and 183 age- and gender-matched normal controls were enrolled. The inclusion criteria were patients with angiographically proven normal coronary arteries and slow flow in at least one of three main coronary arteries (SCFS group, 159 males and 26 females, mean age 46±10 years), and 183 subjects with angiographically proven normal coronary arteries with normal coronary flow ([NCF] group, 162 males and 21 females, mean age 44±8 years) were also included.
All subjects enrolled in this study had normal hepatic and renal function. Current smoking, hypertension, diabetes mellitus, and hyperlipidemia were defined according to past literature reports (4–7). The following exclusion criteria were applied: evidence of coronary artery disease, myocardial infarction, valvular heart disease, congestive heart failure, left ventricular dysfunction, and echocardiographically proven left ventricular hypertrophy, or a history of dysphagia, swallowing and intestinal motility disorders, untreated thyroid disease, sinus node dysfunction or conduction disturbance, estrogen replacement therapy, carcinoma, poorly controlled hypertension (systolic blood pressure >160 mmHg or diastolic blood pressure >105 mmHg), a recent major operation (<3 months), autoimmune disease, or metabolic syndrome. In addition, patients with previous histories of anemia and those who had received previous red blood cell transfusions or were being treated for anemia (e.g., with supplemental iron, folate, or an erythropoiesis-stimulating agent) were not included in this study. Patients with known hematological disease, such as hemolytic anemia, neoplastic metastases to the bone marrow, or iron replacement therapy, which could increase plasma RDW levels, were also excluded.
The baseline characteristics of all enrolled subjects were recorded, including age, gender, body mass index (BMI), diabetes mellitus, hypertension, dyslipidemia, smoking, family history of coronary artery disease, left ventricular ejection fraction, creatinine level, and current medications.
Coronary angiographyElective coronary angiography was performed for all enrolled patients using the standard Judkins technique, and the results were analyzed by at least two interventional physicians, as in our previous study (17). Only angiograms with visually smooth contours with no wall irregularities were considered to be normal. Iopromide was used as a contrast in the angiography (Ultravist-370, Schering AG, Berlin, Germany).
Assessment of coronary blood flowThe coronary flow rates of all subjects were determined by the thrombosis in myocardial infarction (TIMI) frame counts (TFCs) because the method is a simple, reproducible, objective, and quantitative index of coronary flow velocity (18). A TFC was determined for each major coronary artery in each patient and control subject according to the method first described by Gibson et al. (18). Briefly, the number of cineangiographic frames (recorded at 30 frames per second) required for the leading edge of the column of radiographic contrast to reach a predetermined landmark is determined. The first frame is defined as the frame in which the concentrated dye occupies the full width of the proximal coronary artery lumen, touching both borders of the lumen and exhibiting forward motion in the artery. The final frame is designated when the leading edge of the contrast column initially arrives at the distal landmark. In the left anterior descending (LAD) coronary artery, the landmark used is the most distal branch nearest the apex of the left ventricle, commonly referred to as a “pitchfork”. All antianginal and anti-ischemic medications, except for sublingual nitroglycerin, were withheld for at least 24 hours before the examination. To exclude the possibility of a coronary spasm during coronary angiography, all patients underwent hyperventilation tests, which were performed by asking the patients to breathe quickly and deeply for at least five minutes.
The mean TFC for each patient and control subject was calculated by adding the TFCs of the LAD artery, left circumflex artery (LCX), and right coronary artery (RCA) and then dividing the sum by three. All participants with a TFC >27 were diagnosed with SCFS (18). The mean TFCs reported by the two independent observers were compared to assess inter-observer reliability. A discrepancy was subsequently resolved by a third observer. For the first 30 coronary angiograms, the reviewing process was repeated at the end of the study to determine intra-observer variability.
Determinations of RDW and C-reactive protein (CRP)Erythrocyte count, hemoglobin, RDW, corpuscular volume, and white blood cell (WBC) count were determined using the automated hematology analyzer XE-1200 (Sysmex, Kobe, Japan). The normal range of RDW (%) in our laboratory was 10-16%. The other biochemical measurements were performed using a molecular analyzer (Roche Diagnostics, Manheim, Germany).
EDTA-anticoagulated peripheral blood samples were collected after a 12-hour overnight fast at baseline (before the coronary angiography). The plasma was obtained after centrifugation at 3000 rpm at 4°C for 15 minutes. The levels of high-sensitivity CRP were determined using immunoturbidimetry (Beckmann Assay 360), as in our previous study (17). The median normal CRP value was 0.08 mg/dL, with 90% of normal values <0.03 mg/dL and with a lower detection limit of 0.02 mg/dL. The inter-assay and intra-assay coefficients of variation were 4.5% and 5.0%, respectively. Finally, to assess the potential relationship between RDW levels and TFCs or CRP, a correlation analysis was performed for patients with SCFS.
Statistical analysisThe continuous variables are expressed as the mean±standard deviation (SD), and the categorical variables are expressed as percentages. A comparison of continuous variables between the two groups was performed using Student's t-test and/or the Mann-Whitney U-test. The chi-squared test or Fisher's exact test was used to compare the categorical variables between the two groups. Because the CRP distribution is skewed rightward, a log transformation was performed at baseline, and the significance of any difference in the distributions was assessed with the Wilcoxon rank-sum test, as in our previous study (17). Univariate and multivariate analyses were used for the baseline clinical characteristics, inflammatory biomarkers, and RDW. The association between RDW and CRP or TFC was tested using Spearman's correlation coefficient. The SCFS patients were divided into three groups using the upper tertile of the baseline RDW and CRP values as a prespecified cutoff (RDW, 13.8% and CRP, 3 mg/dL). A p-value <0.05 was considered statistically significant.
RESULTSBaseline clinical characteristicsThe baseline clinical characteristics of the SCFS patients (n = 185) or the age- and gender-matched normal controls (n = 183) are summarized in Table 1. There were no significant differences between the SCFS group and the controls in the following baseline clinical variables: age, BMI, family history of coronary artery disease, left ventricle function, the mean diameter of the coronary arteries, and medications. However, the risk factors for cardiovascular disease, such as current smoking, hypertension, dyslipidemia, and diabetes, were higher in the SCFS patients than in the normal controls, although a higher TFC was found in the SCFS patients (Table 1).
Baseline clinical characteristics (mean±SD).
| Variables | SCFS group | NCF group | p-value |
|---|---|---|---|
| (n = 185) | (n = 183) | ||
| Age (years) | 46±10 | 44±8 | 0.312 |
| Male/female | 159/26 | 162/21 | 0.409 |
| Body mass index (kg/m2) | 23±6 | 22±4 | 0.055 |
| Family history of CAD, n (%) | 21 (11.4) | 19 (10.4) | 0.268 |
| Current smoker, n (%) | 46 (24.9) | 28 (15.4) | 0.037 |
| Hypertension, n (%) | 68 (36.8) | 35 (19.1) | 0.013 |
| Dyslipidemia, n (%) | 39 (21.1) | 25 (13.7) | 0.058 |
| Diabetes, n (%) | 15 (8.1) | 7 (3.5) | 0.050 |
| LVEF (%) | 61±9 | 62±6 | 0.182 |
| Medications | |||
| Aspirin, n (%) | 159 (85.9) | 150 (82.0) | 0.493 |
| β-blocker, n (%) | 47 (25.4) | 26 (14.2) | 0.042 |
| ACEI, n (%) | 52 (28.1) | 44 (24.0) | 0.509 |
| Statin, n (%) | 36 (19.5) | 19 (10.4) | 0.186 |
| CCB, n (%) | 23 (17.8) | 17 (9.4) | 0.08 |
SCFS = slow coronary flow syndrome; NCF = normal coronary flow; CAD = coronary artery disease; LVEF = left ventricular ejection fraction; ACEI = angiotensin-converting enzyme inhibitor; CCB = calcium channel blocker.
As shown in Table 2, there were no differences in laboratory variables, including erythrocyte count, mean corpuscular volume, hemoglobin, and creatinine, between the two groups. However, the WBC counts, monocyte counts, and plasma CRP levels were higher in the SCFS patients than in the controls (WBC count, 6836±1130/mm3versus 6327±1092/mm3, p = 0.047; monocyte count, 641±162/mm3versus 609±147/mm3, p = 0.035; CRP level, 0.29±0.16 mg/L versus 0.21±0.14 mg/L, p = 0.042). In addition, the pattern of the RDW levels was similar to the pattern of the CRP levels, with elevated RDW levels in the SCFS patients compared to the controls (13.5±1.8 versus 12.8±1.6, p = 0.031).
Laboratory findings (mean±SD).
| Variables | SCFS group | NCF group | p-value |
|---|---|---|---|
| (n = 185) | (n = 183) | ||
| hs-CRP (mg/dL) | 0.29±0.16 | 0.21±0.14 | 0.042 |
| RDW (%) | 13.5±1.8 | 12.7±1.5 | 0.031 |
| White blood cell count (/mm3) | 6836±1130 | 6327±1092 | 0.047 |
| Monocyte count (/mm3) | 641±162 | 609±147 | 0.035 |
| Erythrocyte count (1012/L) | 4.3±0.6 | 4.3±0.5 | 0.427 |
| Mean corpuscular volume (fL) | 83±4.6 | 85±4.3 | 0.388 |
| Hemoglobin (g/dL) | 13.6±1.4 | 13.5±1.5 | 0.324 |
| Creatinine (mg/dL) | 1.3±0.4 | 1.3±0.4 | 0.562 |
| Vascular diameter (mm) | 3.23±0.36 | 3.21±0.40 | 0.712 |
| TFC | 51.7±15.5 | 38.8±6.4 | 0.018 |
SCFS = slow coronary flow syndrome; NCF = normal coronary flow; Hs-CRP = high-sensitivity C-reactive protein; RDW = red cell distribution width; TFC = thrombolysis in myocardial infarction frame count.
In this study, the seven variables associated with SCFS, including smoking, hypertension, dyslipidemia, diabetes, peripheral circulating WBCs, monocytes, CRP and RDW (p<0.05 in the univariate analysis) are presented in Table 3 (smoking, odds ratio [OR] 1.354, 95% confidence interval [CI], 1.081-1.593, p = 0.031; diabetes, OR 1.286, 95% CI, 1.052-1.476, p = 0.046; hypertension, OR 1.277, 95% CI, 1.048-1.432, p = 0.050; WBCs, OR 1.287, 95% CI, 1.071-1.840, p = 0.051; monocytes, OR 1.426, 95% CI, 1.157-1.885, p = 0.041; CRP, OR 1.901, 95% CI, 1.292-3.127, p = 0.015; RDW, OR 1.962, 95% CI, 1.319-3.348, p = 0.003). We then included these seven variables in a multivariate analysis. Interestingly, we found that RDW (tertile, >13.8%) and CRP (tertile, >3 mg/dL) were the independent variables most strongly associated with SCFS (RDW, OR 3.973, 95% CI, 1.352-4.262, p = 0.001; CRP, OR 3.112, 95% CI, 1.226-3.633, p = 0.018; Table 4.
Multivariate analysis of variables linked to SCFS.
| Variables | Chi-squared test | p value | OR | 95% CI |
|---|---|---|---|---|
| Smoking | 3.283 | 0.031 | 1.354 | 1.081-1.593 |
| Diabetes | 3.057 | 0.046 | 1.286 | 1.052-1.476 |
| Hypertension | 2.974 | 0.050 | 1.277 | 1.048-1.432 |
| WBC count | 3.127 | 0.051 | 1.287 | 1.071-1.840 |
| PMC | 3.628 | 0.041 | 1.426 | 1.157-1.885 |
| CRP | 4.201 | 0.015 | 1.901 | 1.292-3.127 |
| RDW | 4.831 | 0.003 | 1.962 | 1.319-3.348 |
SCFS = slow coronary flow syndrome; WBC = white blood cell; PMC = peripheral monocyte count; CRP = C-reactive protein; RDW = red cell distribution width.
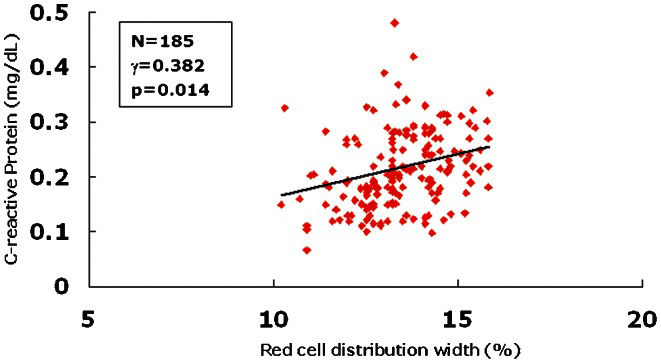
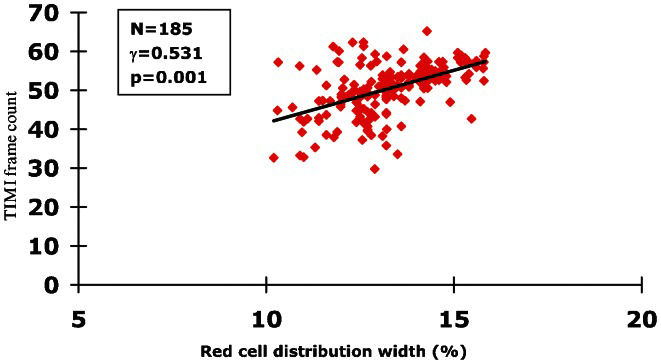
A linear correlation analysis was first performed to determine the relationship between the RDW and CRP levels in the SCFS patients. In addition, the correlation between the RDW and mean TFCs was also analyzed. A significantly positive correlation between the RDW levels and CRP (Figure 1), n = 185, γ = 0.382, p = 0.014) or mean TFCs (Figure 2), n = 185, γ = 0.531, p = 0.001) was detected.
In the present study, we demonstrated for the first time that higher RDW levels exist in SCFS patients. In addition, the data showed that baseline RDW levels are an independent predictor of this unique disorder. Finally, our study indicated that there is a strongly positive correlation between the RDW levels and CRP or TFCs in patients with SCFS. Taken together, the results of the present study indicate that RDW may be a useful marker for detecting the presence of SCFS.
SCFS was first described as early as 1972. A limited number of studies have focused on the etiology of this uncommon angiographic phenomenon since that time, and the pathophysiological mechanism of SCFS remains largely unclear; however, several potential hypotheses have been suggested, such as the organic or functional dysfunction of small coronary arteries, an earlier form of atherosclerosis, platelet aggregability, and an imbalance between vasoconstricting and vasodilating factors (3). Inflammation has been considered to be a major contributing factor in many cardiovascular events and is associated with different clinical settings of coronary artery disease, which may be involved in SCFS development. In fact, Turhan et al. found that serum intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin levels were higher in SCFS patients than in NCF patients. Moreover, these increased soluble ICAM-1, VCAM-1, and E-selectin levels were significantly correlated with average TFCs in SCFS patients (19). Our previous study also demonstrated that increased plasma IL-6 levels were related to the presence of SCFS (20). Therefore, we hypothesized that SCFS might be a syndrome that is strongly associated with an inflammatory response (3,20).
An increased RDW results from heterogeneity in the size of erythrocytes and erythrocyte fragmentation in the circulation. The RDW is generally used in combination with the mean corpuscle volume as an indicator in the differential diagnosis of anemia (21). Factors that contribute to the increased heterogeneity of erythrocyte size include iron or vitamin B12/folate deficiency, decreased erythrocyte lifespan, impaired erythropoiesis, and factors that contribute to erythrocyte fragmentation, including increased fragility and the destruction of red blood cells (22,23). Recently, an elevated RDW was found to be a strong and independent predicator of an increased risk of mortality and adverse cardiovascular outcomes in patients with acute and chronic cardiac conditions (4–16). Although these studies demonstrated the role of RDW in a variety of cardiovascular diseases, the association between RDW levels and SCFS has not yet been assessed. In the present study, we elaborated on the results of previous studies and found, for the first time, that RDW is significantly higher in SCFS patients than in normal controls (13.5±1.8 versus 12.8±1.6, p = 0.031). A multivariate analysis showed that the upper tertile of RDW (>13.8%) was an independent predictor of SCFS (OR 3.973, 95% CI 1.352-4.262, p = 0.018). Our data may provide information regarding the role of RDW in cardiovascular diseases.
The exact mechanism underlying the correlation between higher levels of RDW and the presence of SCFS is relatively unknown. One of the most likely mechanisms is inflammation. In fact, recent findings have suggested that inflammation may play a pathogenic role in SCFS (3). A higher RDW was associated with inflammatory markers, such as soluble tumor necrosis factor receptors and CRP, in atherosclerosis and other chronic diseases (21,22). A strong association between RDW and inflammatory markers was found in a large cohort of unselected adult outpatients and in patients with inflammatory bowel disease (23). Proinflammatory cytokines may also contribute to the heterogeneity of the erythrocyte population through other pathways, such as oxidative stress (24). Red blood cells have a large antioxidant capacity and serve as a primary oxidative sink, but these cells are prone to oxidative damage, which reduces cell survival and induces the release of juvenile erythrocytes into the circulation (25). In the present study, the data first demonstrated that elevated plasma CRP was higher in the SCFS patients compared with the normal controls (0.29±0.16 mg/L versus 0.21±0.14 mg/L, p = 0.042). A positive correlation between RDW and plasma CRP or TFCs was then found in the SCFS patients, thereby suggesting that an inflammatory mechanism may be involved in higher RDW levels in SCFS patients.
In conclusion, in this prospective, age- and gender-matched case-control study, the data demonstrated, for the first time, that RDW levels are higher and positively correlated with CRP and TFC in SCFS patients. This finding suggests that RDW may be a useful marker and predictor for SCFS.
LIMITATIONSThe small sample size may be a study limitation. Other factors, including iron, vitamin B12, and folate, were not measured in this study.
AUTHOR CONTRIBUTIONSLuo SH and Jia YJ collected the data and prepared the manuscript. Li JJ designed the experiments, interpreted the data, and revised the manuscript. Nie SP, Qing P, Guo YL, Liu J, Xu RX, Zhu CG, Wu NQ, Jiang LX, Dong Q, and Liu G collected the data.
This research was partially supported by the National Natural Scientific Foundation (81070171), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20111106110013), and the Fund of Capital Special Foundation of Clinical Application Research (Z121107001012015), which was awarded to Jian-Jun Li, MD, PhD.
No potential conflict of interest was reported.