The net effects of acute normovolemic hemodilution with different hemoglobin levels on splanchnic perfusion have not been elucidated. The hypothesis that during moderate and severe normovolemic hemodilution, systemic and splanchnic hemodynamic parameters, oxygen-derived variables, and biochemical markers of anaerobic metabolism do not reflect the adequacy of gastric mucosa, was tested in this study.
METHODS:Twenty one anesthetized mongrel dogs (16 ± 1 kg) were randomized to controls (CT, n = 7, no hemodilution), moderate hemodilution (hematocrit 2 5% ± 3%, n = 7) or severe hemodilution (severe hemodilution, hematocrit 15% ± 3%, n = 7), through an isovolemic exchange of whole blood and 6% hydroxyethyl starch, at a 20 mL/min rate, to the target hematocrit. The animals were followed for 120 min after hemodilution. Cardiac output (CO, L/min), portal vein blood flow (PVF, mL/min), portal vein-arterial and gastric mucosa-arterial CO2 gradients (PV-artCO2 and PCO2 gap, mm Hg, respectively) were measured throughout the experiment.
RESULTS:Exchange blood volumes were 33.9 ± 3.3 and 61.5 ± 5.8 mL/kg for moderate hemodilution and severe hemodilution, respectively. Arterial pressure and systemic and regional lactate levels remained stable in all groups. There were initial increases in cardiac output and portal vein blood flow in both moderate hemodilution and severe hemodilution; systemic and regional oxygen consumption remained stable largely due to increases in oxygen extraction rate. There was a significant increase in the PCO2-gap value only in severe hemodilution animals.
CONCLUSION:Global and regional hemodynamic stability were maintained after moderate and severe hemodilution. However, a very low hematocrit induced gastric mucosal acidosis, suggesting that gastric mucosal CO2 monitoring may be useful during major surgery or following trauma.
Os efeitos da hemodiluição normovolêmica com diferentes níveis de hemoglobina na perfusão esplâncnica são pouco conhecidos. Testamos a hipótese que durante a hemodiluição moderada e acentuada, os parâmetros hemodinâmicos sistêmicos e regionais e as variáveis relacionadas ao metabolismo de oxigênio não refletem a adequação da perfusão da mucosa gástrica.
MÉTODOS:Vinte e um cães anestesiados com fentanil e vecurônio (16±1 kg) foram randomizados como controles (CT, n=7, sem hemodiluição normovolêmica), hemodiluição normovolêmica moderada (Ht 25±3%, n=7) ou hemodiluição normovolêmica acentuada (Ht 15±3%, n=7), pela troca isovolêmica entre o sangue total e hidroxietil amido a 6%, 20 mL/min até o hematócrito pré-estabeleci-do para cada grupo. Os animais foram acompanhados por 120 min após a hemodiluição normovolêmica. Durante todo o experimento foram medidos o débito cardíaco (CO, L/min), o fluxo de veia porta (PVF, mL/min), e os gradientes de CO2 veia porta-arterial e mucosa gástrica-arterial (PV-artCO2 and PCO2-gap, mmHg, respectivamente).
RESULTADOS:O volume de sangue trocado foi de 33,9±3,3 mL/kg para hemodiluição normovolêmica moderada e de 61,5±5,8 mL/kg para a hemodiluição normovolêmica acentuada. A pressão arterial e os níveis de lactato sistêmico e regional permaneceram estáveis em todos os grupos. Houve aumentos iniciais de débito cardíaco e de fluxo de veia porta, tanto na hemodiluição normovolêmica moderada quanto na hemodiluição normovolêmica acentuada; o consumo de oxigênio sistêmico e regional permaneceram estáveis, principalmente por conta de aumentos na taxa de extração de oxigênio. O PCO2-gap apresentou aumento significativo apenas nos animais submetidos a hemodiluição normovolêmica acentuada.
CONCLUSÃO:Ocorre estabilidade hemodinâmica global e regional tanto na hemodiluição normovolêmica moderada quanto na acentuada. Entretanto, o hematócrito de 15% induziu acidose moderada de mucosa gástrica, o que pode ser relevante em procedimentos cirúrgicos de grande porte ou no trauma.
The increasing frequency of complex surgical procedures with extensive blood loss, the high costs of transfusion therapy, the shortages in blood banks and the risk of transmission of diseases such as acquired immune deficiency syndrome and hepatitis have increased interest for the intraoperative use of acute normovolemic hemodilution (ANH).1,2
Acute normovolemic hemodilution refers to blood removal and simultaneous replacement with acellular fluids immediately before surgical procedures. Hemodilution could be classified according to the target hematocrit (Ht) as mild (hematocrit ≥ 30%), moderate (30 < hematocrit ≥ 20%), or severe (hematocrit < 20%).3 A decrease in hemoglobin levels after ANH results in reduction of arterial oxygen content and a compensatory increase in cardiac output. However, a decrease in tissue oxygen delivery, particularly to splanchnic organs, is a possible undesirable by-product.
Hypoperfusion of the splanchnic bed has been implicated as the trigger of systemic inflammatory response due to bacterial or toxin translocation from the gut lumen to blood stream, and this phenomenon has been associated with the development of postoperative multiple organ dysfunction and to a worsened outcome.4 The net effects of ANH with different hemoglobin levels on splanchnic perfusion have not been elucidated. We tested the hypothesis that during moderate and severe normovolemic hemodilution, systemic and splanchnic hemodynamic parameters, oxygen-derived variables, and biochemical markers of anaerobic metabolism do not necessarily reflect the adequacy of gastric mucosal perfusion, which may be impaired.
MATERIALS AND METHODSThe experimental protocol was approved by the Ethical Review Board of the Institution with adherence to the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and to the “Guide for the Care and Use of Animals” by the National Institutes of Health.
Animal preparationThe present study was performed using 21 male mongrel dogs, weighing 16.7 ± 0.7 kg. The animals were fasted for 12 hours before the study, with free access to water. Animals with a hematocrit below 30% were excluded. Anesthesia was induced with an intravenous injection of sodium pentobarbital, 25 mg/kg. After endotracheal intubation, the animals were mechanically ventilated. The ventilator was adjusted to achieve an initial arterial PCO2 between 35 and 45 mm Hg, with a 1.0 inspired oxygen fraction throughout the experiment. Anesthesia was maintained with intravenous injections of fentanyl (0.005 mg/kg/h) and vecuronium bromide (0.15 mg/kg/h).
The right common femoral vein was catheterized for measurement of inferior vena cava pressure, for infusion of Ringer’s solution (4 mL/kg/h) during the experiment, and for volume replacement during hemodilution. A polyethylene cannula was inserted into the right common femoral artery for blood removal. The left common femoral artery was catheterized to measure the mean arterial pressure (MAP) and to collect samples for blood gas, pH, bicarbonate, base deficit, hematocrit, and hemoglobin analysis.
A 7.5 Fr flow-directed thermodilution fiberoptic pulmonary artery catheter with thermal filament (CCOmbo 744H7.5F, Edwards Swan-Ganz, Edwards Life and Sciences, Irvine, CA, USA) was introduced through the right external jugular vein and its tip placed into the pulmonary artery, guided by pressure monitoring and wave tracings. This catheter was connected to a cardiac computer (Vigilance, Edwards Life and Sciences) to measure mean pulmonary arterial pressure and cardiac output and to collect mixed venous samples for blood gas analysis. All pressure-measuring catheters were connected to pressure transducers (Transpac Disposable Transducer, Abbott, Chicago, IL, USA) and then to a Biopac Data Acquisition System (Model MP100, Biopac Systems, Goleta, CA, USA) for continuous recording of systemic and pulmonary artery pressures.
Through a median laparotomy, the spleen was removed, and a polyethylene catheter was placed into the portal vein, through the splenic vein, to draw blood samples and monitor portal vein pressure. A transit time ultrasonic flowprobe (Transonic System Inc., Ithaca, NY, USA) was positioned around the portal vein and connected to a flowmeter (T206 Transonic Volume Flowmeter, Transonic Systems, Inc, Ithaca, NY, USA).
An orogastric tube was inserted into the stomach, and gastric lavage was performed using warm isotonic saline solution, until a clear fluid solution was obtained. A 16F TRIP® tonometry catheter was then introduced orally and manually placed along the greater gastric curvature. This catheter was connected to a gas capnometer (Tonocap, model TC-200, Tonometrics, Datex-Egstrom, Finland) for gastric PCO2 measurement.
Hemodilution was produced progressively by removing blood from the right common femoral artery at a rate of 20 mL/min while replacing it with 6% hydroxyethyl starch (Plasmasteril, Fresenius Laboratórios Ltda., São Paulo, Brazil) pumped into the left common femoral vein at the same rate.
The total volume removed (TVR) necessary to obtain a given hematocrit (dHct) was calculated using the initial hematocrit (iHct) according to the following formula: TVR (mL) = [70 mL x weight (kg)] x [(iHct-dHct)/(iHctc+dHct/2)]
A microhematocrit sample was obtained every 5 minutes during the hemodilution to check exactly whether the desired hematócrito was attained.
Experimental designAfter the completion of surgical preparation, the animals were allowed to stabilize for 30 minutes. Baseline measurements were obtained, and the animals were randomly assigned to 3 experimental groups: CT (control, n = 7), MHD (moderate normovolemic hemodilution, n = 7, hematocrit = 25% ± 3%) and SHD (severe normovolemic hemodilution, n = 7, hematocrit = 15% ± 3%).
The animals were followed for 120 minutes after hemodilution and were euthanized by anesthesia overdose and hypertonic potassium chloride solution injection.
Measured variablesMean systemic and pulmonary arterial pressures, heart rate, and mesenteric artery blood flow were continuously recorded. Cardiac output was determined using 3-mL bolus injections of isotonic saline at 20oC. Each determination was the arithmetic mean of 3 consecutive measurements when their differences did not exceed 10%.
Arterial, portal, and mixed venous base deficit, pH, PCO2, oxygen tension, oxygen saturation, hemoglobin, hematocrit, and bicarbonate levels, were obtained at baseline (BL), immediately after the end of hemodilution (HD0), and 30, 60, 90, and 120 minutes after hemodilution. All arterial, venous, and portal blood samples were analyzed by a Stat Profile Ultra Analyzer (Nova Biomedical, Waltham, MA, USA).
Systemic and pulmonary vascular resistance (SVR and PVR), oxygen delivery and consumption (DO2 and VO2), and the systemic and splanchnic oxygen extraction ratios (O2ERsyst and O2ERsplanc) were calculated using standard formulae.
Gastric mucosal PCO2 (PrCO2) was evaluated every 10 minutes. PCO2 gap was calculated as the difference between gastric mucosal and arterial PCO2.
Statistical methodologyResults are expressed as mean ± standard error of mean (SEM). Statistical analysis was performed using a Statistic Package for Social Sciences for Windows software (version 6.0, SPSS Inc., Chicago, IL, USA).
Differences between groups were analyzed using repeated measures analysis of variance and post hoc Tukey’s test. Statistical significance was considered for P values less than 0.05.
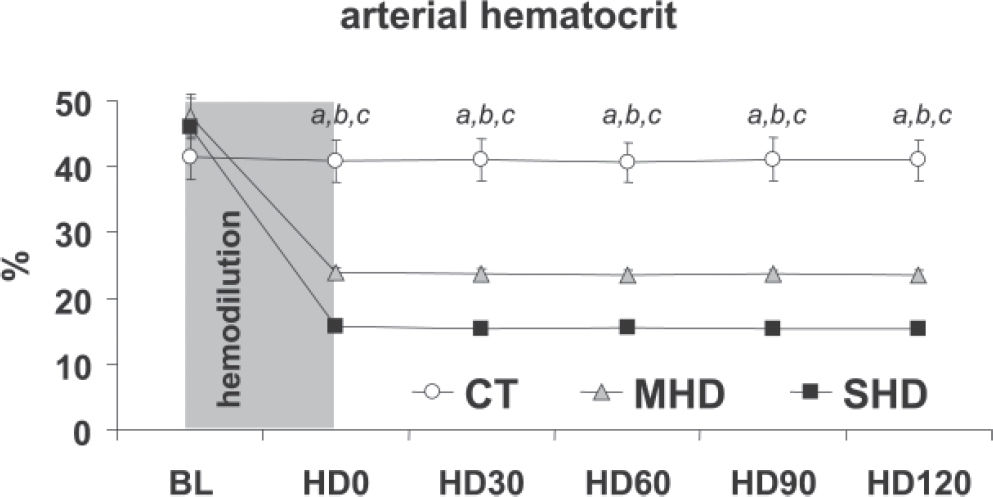
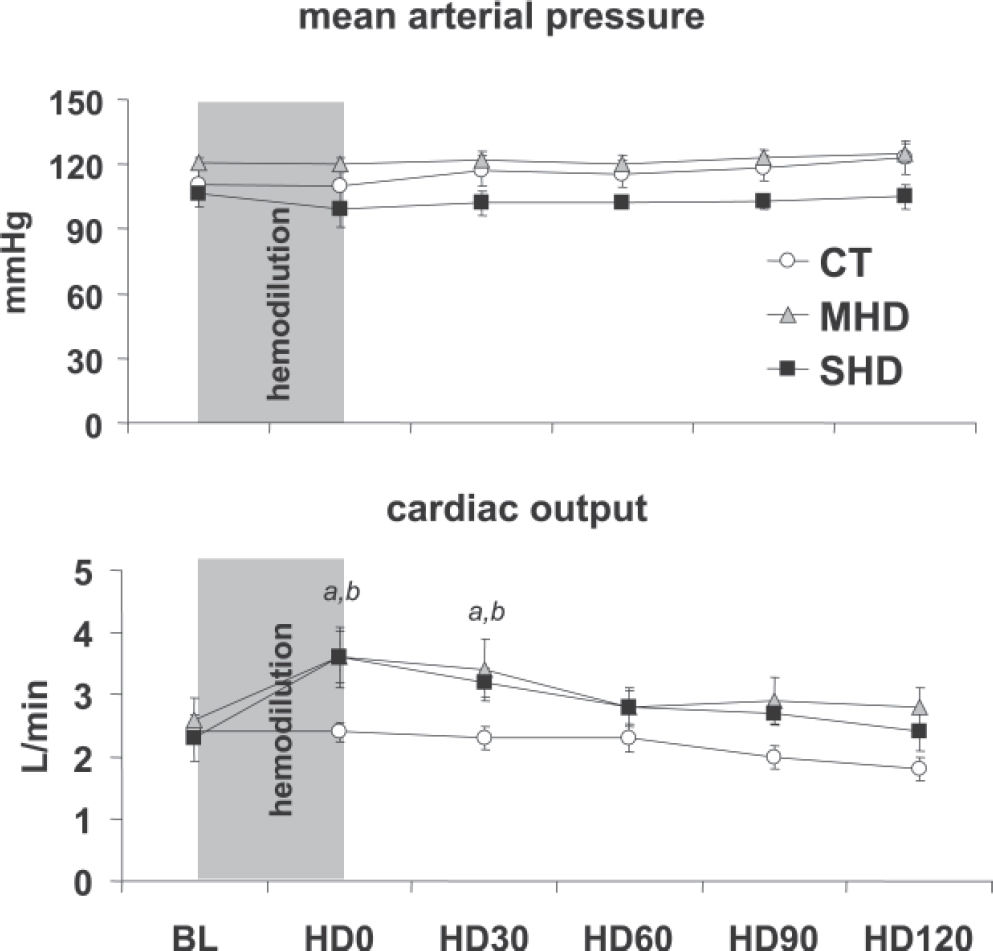
RESULTSBaseline measurements were similar between groups. Both moderate and severe acute normovolemic hemodilution promoted a sustained and significant reduction in hematocrit levels, as planned (Figure 1). Mean arterial pressure (Figure 2A), central venous, and pulmonary wedge pressures (not shown) showed no significant changes throughout the experimental protocol in all groups.
(A) Mean arterial pressure during the experiment in the control (CT), moderate hemodilution (MHD), and severe hemodilution (SHD) groups. No significant changes between and within groups were observed. (B) Cardiac output during the experiment in the control (CT), moderate hemodilution (MHD) and severe hemodilution (SHD) groups. (a) MHD vs CT, P < 0.01; (b) SHD vs CT, P < 0.01.
There was a significant increase in cardiac output after hemodilution in both MHD and SHD up to time point HD30; thereafter, this difference was no longer significant (Figure 2B).
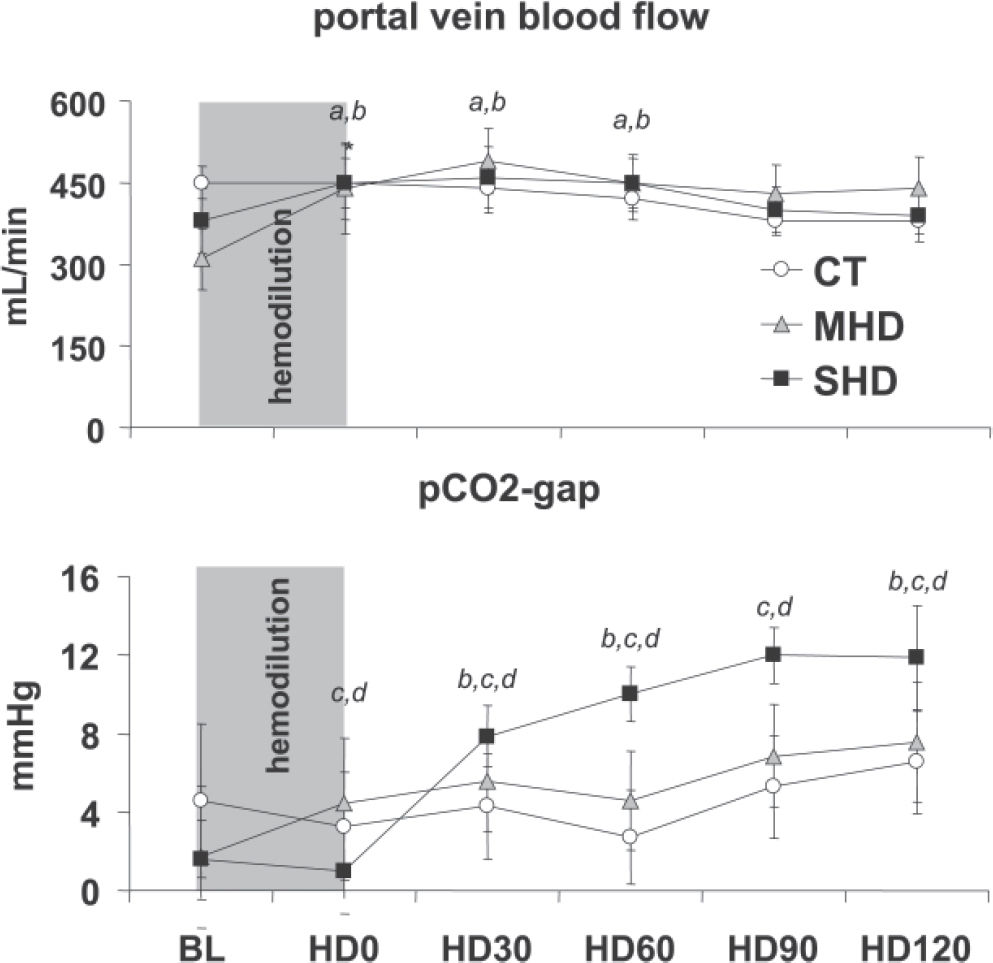
Hemodilution promoted a transient significant increase in portal vein blood flow in comparison to baseline values in the MHD and SHD groups at HD0, HD30, and HD60. However, there was no significant difference between groups when considered for the entire experimental time. (Figure 3A).
(A) Portal vein blood flow during the experiment in the control (CT), moderate hemodilution (MHD), and severe hemodilution (SHD) groups. (a) MHD vs CT, P < 0.05; (b) SHD vs CT, P < 0.05. (B) PCO2 gap during the experiment in the control (CT), moderate hemodilution (MHD), and severe hemodilution (SHD) groups. (d) SHD vs CT, P < 0.05; (c) MHD vs SHD, P < 0.05; (b) SHD vs CT, P < 0.05.
Systemic and regional oxygen-derived variables, pH and base excess, as well as lactate levels are depicted in Tables 1 and 2, respectively. Control animals showed no significant changes during the entire experiment. Hemodilution promoted a sustained decrease in systemic and regional oxygen delivery, which was significantly greater in the SHD than in the MHD group. Systemic and regional oxygen consumption remained stable through increases in oxygen extraction rate. Additionally, systemic and regional lactate levels did not rise in the 3 groups.
Systemic oxygen delivery, consumption and extraction (DO2, VO2 and O2ER, respectively) and arterial pH, base excess and lactate, in control (CT) moderate and severe hemodilution (MHD and SHD) groups
| GROUPS | BL | HD0 | HD30 | HD60 | HD120 | |
|---|---|---|---|---|---|---|
| Systemic DO2 | CT | 433± 26 | 442±32 | 398±43 | 414±49 | 318±30 |
| MHD | 535± 86 | 416±55a,b | 384±63a,b | 314±27a,b | 304±32a,b | |
| SHD | 518±111 | 326±52a,b | 271±22a,b | 236±23a,b | 202±30a,b | |
| Systemic VO2 | CT | 75±11 | 80± 9 | 55± 3 | 73± 7 | 61± 9 |
| MHD | 68± 8 | 55±17 | 79±13 | 69±10 | 50±18 | |
| SHD | 87±14 | 76±10 | 83± 6 | 70±10 | 78± 8 | |
| Systemic O2ER | CT | 18±3 | 18±2 | 15±1 | 18±2 | 20±3 |
| MHD | 14±2 | 14±5 | 21±4 | 23±4 | 20±7 | |
| SHD | 19±4 | 27±4a,b,c | 32±3 a,b,c | 30±3 a,b,c | 41±5 a,b,c | |
| arterial pH | CT | 7.43±0.02 | 7.44±0.01 | 7.42±0.01 | 7.43±0.01 | 7.45±0.01 |
| MHD | 7.40±0.03 | 7.41±0.01 | 7.41±0.01 | 7.43±0.01 | 7.43±0.01 | |
| SHD | 7.40±0.02 | 7.42±0.01 | 7.43±0.01 | 7.43±0.01 | 7.43±0.01 | |
| arterial BE | CT | 1.86±0.62 | 1.36±0.40 | 2.14±0.46 | 1.87±0.65 | 1.26±0.41 |
| MHD | 1.03±0.34 | 0.80±0.25 | 0.61±0.13 | 0.91±0.35 | 0.84±0.28 | |
| SHD | 1.14±0.19 | 1.76±0.50 | 1.30±0.35 | 0.93±0.30 | 1.41±0.16 | |
| Systemic Lactate | CT | 1.2±0.1 | 1.1±0,1 | 1±0.2 | 0.8±0.2 | 0.9±0.2 |
| MHD | 1.3±0.4 | 1.1±0.3 | 1.3±0.2 | 1.1±0.3 | 1.1±0.3 | |
| SHD | 1±0.3 | 0.5±0.2 | 0.7±0.2 | 0.8±0.2 | 0.8±0.3 |
Legends: (a) p<0.05 vs. CT; (b) p<0.05 vs. BL; (c) p<0.05 vs. MHD
Splanchnic oxygen delivery, consumption and extraction (DO2, VO2 and O2ER, respectively) and portal vein lactate levels, in control (CT) moderate and severe hemodilution (MHD and SHD) groups.
| GROUPS | BL | HD0 | HD30 | HD60 | HD120 | |
|---|---|---|---|---|---|---|
| Splanchnic DO2 | CT | 82±9.0 | 83±11.3 | 78±9.4 | 75±7.5 | 66±4.6 |
| MHD | 62±9.3 | 51±9.6a,b | 54±7.3a,b | 50±6.6a,b | 48±6.6a,b | |
| SHD | 84±17.9 | 41±6.8a,b | 40±5.6a,b,c | 38±4.5a,b,c | 32±4.9a,b,c | |
| Splanchnic VO2 | CT | 8±1 | 9±1 | 6±1 | 8±1 | 10±1 |
| MHD | 10±3 | 6±2 | 5±2 | 11±3 | 8±1 | |
| SHD | 12±3 | 6±2 | 6±2 | 7±1 | 6±1 | |
| Splanchnic O2ER | CT | 10±2 | 11±2 | 9±1 | 11±1 | 16±2 |
| MHD | 16±3 | 17±7 | 11±4 | 22±5 | 19±5 | |
| SHD | 16±4 | 16±5 | 16±4 | 20±3 | 22±5 | |
| Portal vein Lactate | CT | 1.3±0.1 | 1.2±0.1 | 0.8±0.2 | 1±0.1 | 1.1±0.2 |
| MHD | 1.4±0.4 | 1.1±0.3 | 1.4±0.3 | 1.4±0.3 | 1.2±0.3 | |
| SHD | 1.1±0.3 | 0.7±0.3 | 0.8±0.3 | 0.8±0.2 | 0.9±0.3 |
Legends: (a) p<0.05 vs. CT; (b) p<0.05 vs. BL; (c) p<0.05 vs. MHD
There was no significant increase in the PCO2 gap in the CT and MHD groups (P = 0.207). In contrast, there was a significant increase in the PCO2 gap in the SHD group (Figure 3B).
DISCUSSIONThe main finding of this study, which is part of a general program of experimental analysis of fluid replacement5,6 was that both moderate and severe hemodilution are associated with regional and systemic hemodynamic stability. However, severe hemodilution, to a hematocrit of 15%, induces moderate gastric mucosal acidosis and an increased PCO2 gap. These findings may become relevant in the presence of a transient hemodynamic instability that may be induced by an intraoperative blood loss or surgical maneuvers such as aortic unclamping and major cancer resections.
Our experimental protocol promoted a predicted hematocrit reduction to the desired target levels, while isovolemia was adequately maintained, as indicated by central venous and pulmonary wedge pressures. With unchanged cardiac filling pressures, arterial pressure was kept stable due to an increase in cardiac output, largely related to the reduction in systemic vascular resistance by the lower viscosity. These compensatory hemodynamic changes are expected.7 However, if a more severe anemia is induced, impairment of myocardial oxygenation could jeopardize cardiac performance.8 In this situation, the compensatory increment of cardiac output may not occur and consequently arterial pressure falls.
The mechanisms that increase cardiac output are also responsible for the increased portal venous blood flow, which was able to parallel cardiac output only because there was no hypovolemia. This fact is important in terms of blood flow distribution during normovolemic hemodilution.
In our experimental model, the critical oxygen delivery level to the various regional territories was probably not achieved. While systemic oxygen consumption did not vary significantly in the splanchnic region, it showed a trend toward reduction, from 12 to 6 mL/kg/min. As extensively demonstrated, oxygen consumption decreases whenever critical oxygen delivery levels are not achieved.9 However, systemic and portal vein lactate levels were kept below 2 mmol/L, suggesting that anaerobic metabolism was not present. Lactate levels only increase when anaerobic metabolism is the main source of cellular energy and/or when there is an impairment of lactate clearance.10 As the portal vein blood flow was normal or even increased, lactate clearance was maintained and, as already mentioned, the level of hemoglobin was not sufficient to trigger anaerobic metabolism. Indeed, the level of venous oxygen saturation was higher than those related to critical oxygen delivery in models of normovolemic hemodilution.11,12 By the end of the experiment, the oxygen extraction rate was around 40% (or oxygen venous saturation around 60%) in severe hemodilution. In a model of decreased cardiac output, the mean oxygen venous saturation that corresponded to the critical point in which VO2 becomes dependent on DO2 was around 25%.13 Hence, hemoglobin levels of about 5 mg/dL are still tolerated without evidence of anaerobic metabolism.11
Hypoperfusion of the splanchnic vascular bed has been implicated as the trigger of systemic inflammatory response and multiple organ dysfunction syndrome, which commonly compromise the outcome after trauma or extensive surgeries.14,15 A decrease in splanchnic DO2 caused by hemodilution could amplify the harmful consequences of tissue perfusion heterogeneity during complex surgeries. The balance between oxygen delivery and consumption in the splanchnic area could be assessed by the arterial and portal oxygen saturation gradients, as well as by the portal lactate levels. Alternatively, the portal-arterial pCO2 gradient could be a surrogate marker of splanchnic blood flow and/or perfusion.16,17 As the portal blood flow was maintained throughout the experiment, the splanchnic oxygen delivery decreased exclusively because hemoglobin dropped. However, as we have observed in the systemic oxygen-derived variables, these hemoglobin levels were not low enough to induce anaerobic metabolism. Additional support for this evidence is the fact that splanchnic oxygen consumption was maintained and portal lactate levels did not increase.
In contrast, the behavior of the gastric mucosal-arterial PCO2 gradient was different. Indeed, the PCO2 gap did not increase significantly during the moderate isovolemic hemodilution, but did during severe isovolemic hemodilution. Additionally, after HD30, all subsequent time points showed significant increases in comparison to baseline. This alteration in the SHD group was not paralleled by splanchnic DO2, VO2, nor by portal vein blood flow, or by portal lactate levels. There are some explanations for this finding. Tissue PCO2 is linearly related to tissue blood flow over a large range of flows, especially if hematocrit remains stable. When tissue blood flow decreases, tissue PCO2 increases simply because the CO2 removal rate decreases, particularly during aerobic metabolism.18,19 However, if tissue blood flow is kept normal, or even if it is increased, tissue CO2 removal can be affected by the hemoglobin level. For the same blood flow, but in the presence of a lower tissue hematocrit, CO2 removal can decrease. This is the first explanation for the greater PCO2 gap observed in the present experiment. Secondly, severe isovolemic hemodilution may have induced anaerobic metabolism at the mucosal layer. Although portal lactate levels have not increased, this fact does not rule out the participation of anaerobic tissue CO2 production. In fact, there is no relationship between splanchnic metabolism and mucosal perfusion.20 Even when the gastric mucosal perfusion is severely compromised, portal vein lactate levels could be normal, basically because the other tissues (muscular and/or serosa) could maintain aerobic metabolism. However, when tissue DO2 falls below the critical point, there is a decrease in CO2 production by aerobic tissue, which counterbalances the anaerobically generated CO2. From our data, it is not possible to exclude the anaerobic CO2 generation, since we did not measure mucosal PO2. However, it has been shown that the main component of the PCO2 gap is flow, since in models of hypoxic hypoxia, the PCO2 gap is normal.21
Dubin et al performed a similar analysis in a sheep model of isovolemic hemodilution or hemorrhage.22 They induced a more severe isovolemic hemodilution, down to a 5% hematocrit. At this point, the PCO2 gap reached circa 15 mm Hg. As the authors compared this PCO2 gap with a 40 mm Hg PCO2 gap after hemorrhage, thereby reducing blood flows, they concluded that severe isovolemic hemodilution is unable to reflect intestinal dysoxia. These results are in agreement with ours. Although we did not induce such an extreme decrease in hematocrit (15% vs. 5%), both models resulted in increased tissue PCO2, suggesting that low hemoglobin levels do not induce tissue dysoxia, but even though there microvascular derangement is serious enough to impair tissue CO2 clearance. In a clinical scenario, there are studies showing that a PCO2 gap greater 15 mm Hg is an early marker of poor prognosis.23,24 In a model of shock and resuscitation, Diebel et al have shown that there is no substantial increase in mucosal PCO2 when a 18% hematocrit was induced after volume resuscitation.25 Although these data apparently are in disagreement to ours, we have noted that it is quite difficult to establish the exact point at which tissue CO2 removal becomes impaired. Different experimental models and resuscitation protocols do interfere with the relationship between flow and hemoglobin. The combination of these factors will probably determine the point at which tissue CO2 accumulation begins. We believe that a direct comparison between these studies is difficult to accomplish due to marked differences between models, ie, normal versus hemorrhaged animals.
Care must be exercised in extrapolating our results to clinical practice. We used normal dogs submitted to a laparotomy and splenectomy and placement of flow probes and catheters. Our follow-up was very short. A long-term study performed in unanesthetized baboons evaluated the effects of withholding transfusion or lowering the transfusion trigger to a hematocrit of 15%, similar to our severe hemodilution model.26 These baboons adapted well to severe anemia, and no adverse effects on long-term survival occurred. We believe that their findings are in agreement to ours, since they used normal baboons submitted to a surgical stress (laparotomy)26 which was similar to that in our dogs. In our acute model, we observed systemic and regional hemodynamic stability, as well as the maintenance of the metabolic status. Only gastric perfusion was moderately affected. We consider this fact our major contribution. We expect that, under a greater surgical stress or trauma, a more significant compromise of splanchnic perfusion may occur in our model as well as in the baboon model.
In spite of these limitations, we believe that our findings may be clinically relevant. Restrictive transfusion policies have been widely recommended to avoid transfusion-related reactions and immunoparalysis.27 Isovolemic hemodilution may be used as a blood-saving technique for several procedures, usually those not commonly associated with hemodynamic instability, such as orthopedic surgeries. Vascular surgeries could be an ideal scenario for isovolemic hemodilution. However, major vascular procedures are associated with abrupt hemodynamic changes and ischemia-reperfusion of large territories. In this scenario, isovolemic hemodilution has been associated with greater hemodynamic instability only during reperfusion.2
In summary, while moderate and severe normovolemic hemodilution are associated with systemic and regional stability, intestinal mucosal hypercarbia was detected after severe normovolemic hemodilution, suggesting impairment of tissue CO2 removal. However, it is unclear from this present experiment whether this phenomenon may be also associated with tissue oxygen compromise that could lead to cellular derangement. Additionally, systemic and splanchnic hemodynamic parameters and biochemical markers of anaerobic metabolism did not reflect the adequacy of gastric mucosal perfusion. These data reinforce the need to monitor regional tissue perfusion in settings of severe hemodilution when larger surgical proceedings are performed.