To analyze the effect of a single bout of resistance exercise on cardiac autonomic modulation in patients with peripheral artery disease.
METHODSFifteen patients with peripheral artery disease (age: 58.3±4.0 years) underwent the following sessions in a random order: resistance exercise (three sets of 10 repetitions of the six resistance exercises with a workload of 5–7 in the OMNI-RES scale) and control (similar to the resistance session; however, the resistance exercises were performed with no load). The frequency domain (low frequency, high frequency and sympathovagal balance) and symbolic analysis (0V, 1V and 2V patterns) of heart rate variability were obtained before and until one hour after the interventions.
RESULTSAfter the resistance exercise and control sessions, similar increases were observed in the consecutive heartbeat intervals (control: 720.8±28.6 vs. 790.9±34.4 ms; resistance exercise: 712.9±30.1 vs. 756.8±37.9 ms; p<0.05) and in the pattern of the symbolic analysis with no variation (0V) (control: 25.1±3.5 vs. 33.4±4.1%; resistance exercise: 26.1±3.2 vs. 29.7±3.5%; p<0.05) until 50 min after both interventions. The pattern of two variations (2V) decreased similarly (control: 11.2±2.1 vs. 8.3±2.1%; resistance exercise: 9.5±1.7 vs. 7.8±1.7%; p<0.05). In contrast, the pattern of one variation (1V), the low and high frequency bands and sympathovagal balance did not change after the interventions (p>0.05).
CONCLUSIONA single bout of resistance exercise did not alter cardiac autonomic modulation in patients with peripheral artery disease.
The autonomic nervous system plays a major role in the regulation of the cardiovascular system (1). Cardiac autonomic modulation is typically assessed based on heart rate variability (HRV) and provides indicators of the relative contributions of the cardiac sympathetic nervous system and parasympathetic autonomic nervous system (2,3). Increases in sympathetic modulation of the heart have been described in pathological conditions (4–6) and are considered to be an important predictor of adverse cardiovascular events (7,8).
Peripheral artery disease (PAD) is a condition that is associated with elevated rates of cardiovascular mortality (9,10). The mechanisms underlying this increased cardiovascular risk are not completely understood but may be related to alterations in cardiac autonomic control. In fact, a previous study (5) observed that PAD patients present increased sympathetic and reduced parasympathetic modulation in the heart, whereas a recent case study indicated that alterations in cardiac autonomic control toward a sympathetic modulation are related to an increased risk of mortality in patients with vasculitis (11). Thus, the analysis of cardiac autonomic control deserves future exploration in PAD patients.
Resistance exercise has been shown to improve walking capacity, quality of life and muscle strength in PAD patients (12–14). In fact, even low-intensity resistance training can improve exercise tolerance in these patients to a similar extent as walking exercise. In addition, low-intensity resistance training increases muscle strength (13,14) and, compared to walking exercise, resistance training produces less leg pain (13). A previous study showed a reduction in blood pressure until one hour after a low-intensity resistance exercise session (15). However, the effects of resistance exercise on cardiac autonomic modulation in these patients are not clear. In healthy subjects, a single bout of moderate-intensity resistance exercise increases cardiac sympathetic modulation and reduces parasympathetic modulation to the heart until one hour after exercise (16–19). In PAD patients with increased sympathetic modulation of the heart at rest (5), this response may be even greater; however, additional studies are needed to confirm this hypothesis.
Because cardiac autonomic responses after resistance exercise are greater when the exercise is performed at higher intensity, in this study we analyzed whether a single bout of low-intensity resistance exercise alters cardiac autonomic modulation in PAD patients. We hypothesized that low-intensity resistance exercise would increase sympathetic modulation and decrease parasympathetic modulation of the heart in these patients.
MATERIAL AND METHODSRecruitmentPatients with PAD were recruited from public hospitals and private vascular clinics. Patients with PAD and symptoms of claudication were included if they: (a) had a graded treadmill test that was limited by claudication, (b) had an ankle brachial index ≤0.90 (c), were nonobese, (d) were not performing any regular exercise program, (e) were not using antihypertensive medications affecting the heart rate (e.g., β-blockers and non-dihydropyridine calcium channel blockers), (f) had systolic blood pressure <160 mmHg and/or diastolic blood pressure <105 mmHg; and (g) had no symptoms related to myocardial ischemia during the treadmill test. Fifteen patients (seven males and eight females) were deemed to be eligible for the study.
Patient screening and preliminary testingAll patients performed a progressive cardiopulmonary treadmill test until they experienced maximal claudication pain, as previously described for these patients (20). During the test, the electrocardiogram was continuously monitored. The claudication onset time and peak walking time were defined, respectively, as the time walked until the patient first reported leg pain and the time at which the patient was unable to continue exercise because of leg pain.
The ankle brachial index at rest was measured as the highest systolic blood pressure in the posterior tibial artery or dorsalis pedis artery divided by the highest systolic blood pressure in the brachial artery (21). The measurement of blood pressure in the ankle and arm were simultaneously measured in triplicate using a Doppler vascular device (Medmega, São Paulo, SP, Brazil) and a mercury sphygmomanometer.
Determination of the loads used in the experimental sessionsPrior to the experiments, the patients underwent familiarization sessions that were designed to standardize the resistance exercises. In each session, the patients executed the following exercises: bench press, knee extension, seated row, knee curl, frontal rise and standing calf raise. In each exercise, they performed three sets of 10 repetitions with the minimum load allowed by the machines.
After familiarization, the patients underwent up to four sessions to identify the load that would be used in the experimental sessions. During these sessions, the load corresponding to a rate of perceived exertion between 5 and 7 in the OMNI resistance exercise scale (22) was determined for each exercise, as previously described (23). Once the workload for an exercise was determined in one session and confirmed in the next session, the exercise was not performed until the experimental sessions.
Experimental protocolThe patients underwent two experimental sessions that corresponded to control (C) and resistance exercise (R) in a random order. Each session was initiated between 7:00 and 8:00 a.m., and the sessions were performed at an interval of seven days. The patients were instructed to eat a light meal before the experiments, to avoid physical exercise and alcohol ingestion for at least 48 hours prior to the session, and to avoid smoking and caffeine for at least 24 hours prior to the session.
In each experimental session, the patients rested in the seated position for 20 min (pre-intervention) in a quiet room. During this period, cardiac autonomic modulation determined using a heart rate monitor (RS 800CX, Polar, USA) in the laboratory by the same observer, who was blinded to which session the subject was going to undergo.
The patients then performed the interventions in an exercise room. The patients were blinded to which session they were going to undergo until the beginning of the intervention. In the R session, the patients performed three sets of 10 repetitions of the six resistance exercises described above with a workload of 5–7 on the OMNI-RES scale. Two-minute intervals were interspersed between the sets and exercises. The C session was similar to the R session; however, the resistance exercises were performed without any load (1 in the OMNI-RES scale).
After the interventions, the patients returned to a quiet room, where they remained seated for 60 min. The cardiac autonomic modulation was obtained two times, between 20–30 and 40–50 min, using the same procedures used for the pre-experimental period.
Cardiac autonomic modulation measurementsCardiac autonomic modulation was determined by frequency-domain and symbolic analysis. The assessment of consecutive heartbeat intervals (RR intervals) was obtained using a heart rate monitor (Polar, RS 800CX, USA).
The frequency-domain variables were analyzed using spectral analysis of HRV. Hence, the stationary periods of the tachogram with at least 500 beats were broken down into bands of low (LF) and high (HF) frequency using the autoregressive method with a model order of 12 according to Akaike's information criterion. Kubios HRV software (Biosignal Analysis and Medical Imaging Group, Joensuu, Finland) was used following the recommendations of the Task Force of Spectral Analysis (3). Frequencies between 0.04 and 0.4 Hz were considered to be physiologically significant, whereas the LF component was represented by oscillations between 0.04 and 0.15 Hz, and HF was represented by oscillations between 0.15 and 0.4 Hz. To interpret the results, the LF and HF normalized components of the HRV were considered, respectively, as representative of predominantly sympathetic and parasympathetic modulation of the heart, and the ratio between these bands (LF/HF) was defined as the cardiac sympathovagal balance (3).
For the nonlinear domain, symbolic analysis (24,25) was performed using SA software (simbolic Analisi, Dipartimento di Scienze Precliniche, Università Degli Studi di Milano, Italy), which transforms the tachogram used in spectral analysis into a sequence of integers (symbols). The full range of the sequences was uniformly spread on six levels (from 0 to 5), and patterns with a length of L = 3 were constructed. All possible patterns were grouped without any loss into three families referred to as patterns with no variation (symbols were equal), patterns with one variation (two consequent symbols were equal, and the remaining symbol was different) and patterns with two variations (all symbols were different from the previous one). The patterns with no variation and two variations were considered, respectively, to be representative of predominantly sympathetic and parasympathetic modulation (24).
Statistical analysesThe Gaussian distribution and the homogeneity of the variance of the data were analyzed by the Shapiro–Wilk and Levene tests, respectively. A dependent t test was used to compare the baseline value between the sessions (C vs. R). To analyze changes in cardiac autonomic modulation variables, a two-way ANOVA for repeated measures was performed. In this analysis, the session factor had two levels (C and R), and the moment factor had three levels (pre-intervention, 20–30 min post-intervention and 40–50 min post-intervention). Newman-Keuls post hoc analyses were performed to determine the significant main and interaction effects.
For all analyses, p<0.05 was accepted as being statistically significant. The data are presented as the mean ± standard error.
EthicsThis study was approved by the Joint Committee on Ethics of Human Research of the University of Pernambuco (process 0134/09). Each patient was informed of the risks and benefits involved in the study and signed a written informed consent before participation.
RESULTSSeven patients initiated the protocol with the C session, and eight patients began with the R session. The characteristics of the sample are presented in Table 1. The majority of the participants were elderly, female, hypertensive and receiving antihypertensive medication.
Physical and functional characteristics of patients with peripheral artery disease (n = 15).
| Values | |
|---|---|
| Age (years) | 58.3±4.0 |
| Weight (kg) | 63.5±2.9 |
| Height (m) | 1.60±0.02 |
| Body mass index (kg/m2) | 25.3±0.9 |
| Ankle brachial index | 0.67±0.03 |
| Initial claudication distance (m) | 235±31 |
| Total walking distance (m) | 654±70 |
| Cardiovascular risk factors | |
| Hypertension (%) | 84.6 |
| Diabetes (%) | 46.2 |
| Medications | |
| Inhibitors of angiotensin-converting enzyme (%) | 30.8 |
| Angiotensin receptor antagonist (%) | 13.4 |
| Calcium channel blocker (%) | 13.4 |
| Diuretics (%) | 23.1 |
The responses of frequency domain variables are presented in Table 2. The pre-intervention cardiac autonomic modulation was similar between the C and R sessions (p>0.05). In comparison with the pre-intervention values, the RR interval increased similarly (p>0.05) after the C and R sessions (p<0.01), whereas the LF, HF and LF/HF did not change in either session (p>0.05).
Cardiac autonomic modulation measured after resistance and control sessions in peripheral artery disease patients (n = 15).
| C session | R session | |||||
|---|---|---|---|---|---|---|
| Pre | 20–30 min | 40–50 min | Pre | 20–30 min | 40–50 min | |
| RR interval (ms) | 720.8±28.6 | 771.0±30.7∗) | 790.9±34.4∗) | 712.9±30.1 | 751.0±37.1∗) | 756.8±37.9∗) |
| Low frequency (nu) | 63.6±4.1 | 65.3±4.8 | 67.0±6.3 | 62.8±3.8 | 67.2±4.4 | 69.0±4.0 |
| High frequency (nu) | 36.4±4.1 | 34.7±4.8 | 33.0±6.3 | 37.2±0.2 | 32.8±4.4 | 31.0±4.0 |
| Low frequency/high frequency | 2.5±0.5 | 3.5±1.0 | 4.2±0.9 | 2.5±0.7 | 3.4±0.9 | 3.4±0.8 |
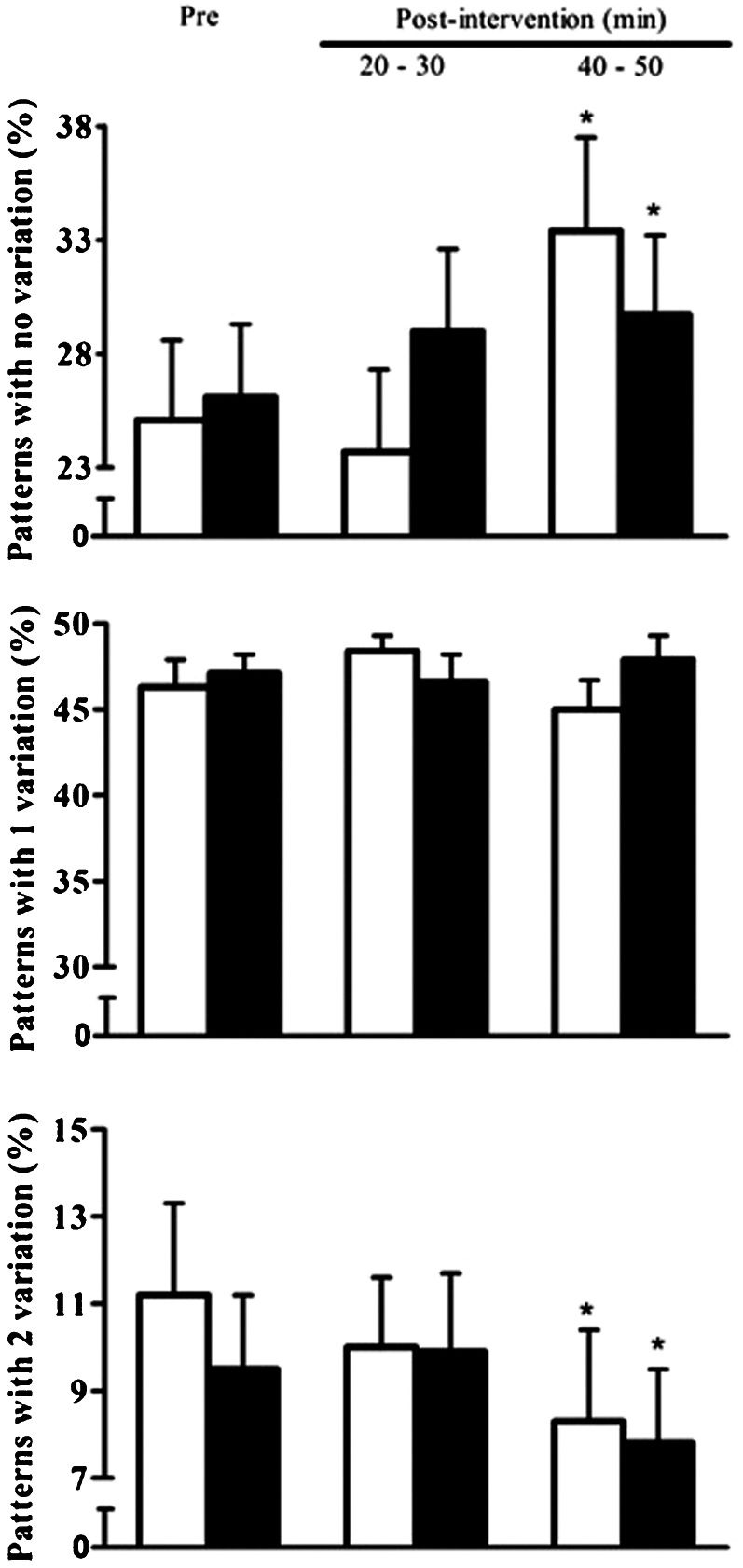
The pre-intervention symbolic analysis variables were similar between the C and R sessions (p>0.05). The responses of the symbolic analysis variables are presented in Figure 1. In comparison with the pre-intervention values, patterns with no variation increased similarly after the C and R sessions only at the 40–50 min recovery period (C – 25.1±3.5 vs. 33.4±4.1%; R – 26.1±3.2 vs. 29.7±3.5%; p<0.05). Patterns with two variations decreased after the C and R sessions at the 40–50 min recovery period (C – 11.2±2.1 vs. 8.3±2.1%; R – 9.5±1.7 vs. 7.8±1.7%; p<0.05). Patterns with one variation did not change after the C and R sessions (C – 46.3±1.6 vs. 45.0±1.7%; R – 47.1±1.1 vs. 47.9±1.4%; p>0.05). For all variables, no differences were found in the response between the C and R sessions (p>0.05).
Patterns with no variation, patterns with one variation and patterns with two variations measured pre- and post-intervention in the control (white bar) and resistance exercise (black bar) sessions in PAD patients (n = 15). ∗Significantly different from the pre-intervention values (p<0.05).
This is the first study to investigate the effects of a single bout of resistance exercise on cardiac autonomic modulation in PAD patients. A major finding is that a single bout of low-intensity resistance exercise promoted autonomic responses similar to those observed after the control session. Considering that resistance exercise training improves the walking capacity, quality of life and muscle strength (12–14) of PAD patients, the maintenance of HRV after the intervention supports the safety of resistance exercise for these patients.
Increased sympathetic and reduced parasympathetic modulation of the heart have been considered to represent an important predictor of fatal and nonfatal cardiac events (7). The results of this study demonstrate that the sympathovagal balance of the PAD patients was 2.5 at baseline, which indicates a shift in cardiac autonomic modulation toward higher sympathetic and lower parasympathetic modulation. This finding is in agreement with a previous study (5) that also observed that HRV indicators of sympathetic and parasympathetic modulation were, respectively, higher and lower in PAD patients than in controls. Altogether, these results suggest that altered cardiac autonomic control is potentially related to the increased cardiovascular mortality observed in PAD patients (9). Thus, future studies should address whether the increased sympathetic modulation of the heart is related to cardiovascular mortality in PAD patients.
Previous studies in healthy subjects have shown that a single bout of resistance exercise affects cardiac autonomic modulation during recovery. Rezk et al. (17) showed that 75 min after a one-repetition-maximum (1-RM) resistance exercise with an intensity of 80%, the sympathetic cardiac autonomic modulation remained elevated in normotensive subjects. Similarly, Lima et al. (18) also observed that in normotensive subjects who underwent a 1-RM resistance exercise session at 70%, cardiac autonomic modulation also remained increased within 60 min of recovery. In contrast, after 1-RM resistance exercise performed at 50%, recovery of cardiac autonomic modulation occurred within 20 min (18). Interestingly, in the present study, cardiac autonomic modulation was similar to that in the control session after 20 min of recovery, which represents a faster recovery than that observed in healthy subjects (16,17).
In this study, cardiac autonomic modulation was evaluated by linear (frequency-domain) and nonlinear (symbolic analysis) methods. Although both methods indicated similar responses between the C and R sessions, the results provided by the linear and non-linear methods were different. In an analysis of the same stationary periods on the tachogram, the linear methods indicated maintenance of cardiac autonomic modulation after both sessions, whereas in the symbolic analysis an increase in cardiac sympathetic modulation was observed. Although both techniques have been considered to be valid for cardiac autonomic assessment (3), nonlinear techniques are more reliable in subjects with arrhythmias (26,27), such as patients with cardiovascular disease (26,28), and provide a better description of cardiac autonomic control (2,3).
In this study, the control session was conducted by performing the exercises without any load. A control session without any exercise may have provided different results. We chose exercises without any load for the control because of our interest in verifying the effect of load on autonomic responses in PAD patients, considering that loading is an important variable for the benefits of strength training (29). This strategy has been adopted in previous studies (11,18) because it has been hypothesized that changing from one exercise to another may produce an effect regardless of the exercise load.
The main strength of the present study is its experimental design, which included a non-exercise control session with a crossover design and the assessment of different indicators of HRV. This design allowed us to determine that some differences between measurements before and after the exercise session were in fact caused by the time course, posture changes or other factors regardless of the exercise load because these differences were also observed in the session without any load. However, our choice to perform the assessment after only a single resistance exercise session can be considered as a weakness because the chronic effects of exercise may have greater clinical relevance, and it is not possible to assume that similar effects would be observed after a single period of resistance training. Therefore, future studies are needed to verify the chronic effects of resistance training on autonomic modulation in these patients.
The present study also has some additional limitations. It did not include a healthy group, and so it was not possible to verify the impact of the disease on cardiac autonomic modulation. However, this objective was not the goal of the present study, and this issue has already been addressed by other authors (15). The patients in this study were using different types of medication, which may have affected the results. To minimize the effects of medication type, we did not include patients using drugs that directly affect the heart rate (e.g., beta-blockers and non-dihydropyridine calcium channel blockers). This study included only patients with PAD and symptoms of intermittent claudication, and the results cannot be extrapolated to patients with other stages of PAD. Finally, low-intensity resistance exercise was employed, and the results of the current study can therefore only be generalized to resistance exercises with similar intensity.
The results of this study show that a single bout of resistance exercise did not alter cardiac autonomic modulation in PAD patients. Future studies should address the chronic effects of resistance exercise on this variable to provide further information regarding the cardiovascular effects of this mode of exercise.
AUTHOR CONTRIBUTIONSLima AH conceived and designed the study, conducted the analyses, drafted the initial manuscript and approved the final version of manuscript for submission. Farah BQ conducted the analyses and approved the final version of the manuscript for submission. Rodrigues LB, Miranda AS, Rodrigues SL and Correia MA performed the data collection and approved the final version of the manuscript for submission. Sobral Filho D conducted medical evaluations of patients and approved the final version of the manuscript for submission. Forjaz CL, Prado WL and Wolosker N contributed to the interpretation of the data and critical evaluation of the manuscript, and approved the final version of the manuscript for submission. Ritti-Dias RM conceived and designed the study, coordinated and supervised the data collection, reviewed, revised the manuscript and approved the final version of the manuscript for submission.
This study was supported by grants of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Programa de Excelência Acadêmica (CAPES - PROEX) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE). RMR, CLM, and WLP would like to thank CNPq for the research scholarship.
No potential conflict of interest was reported.