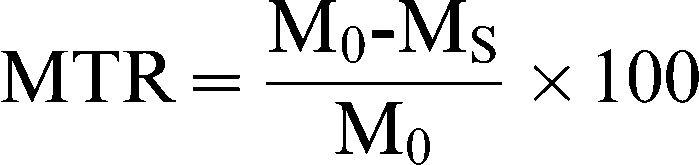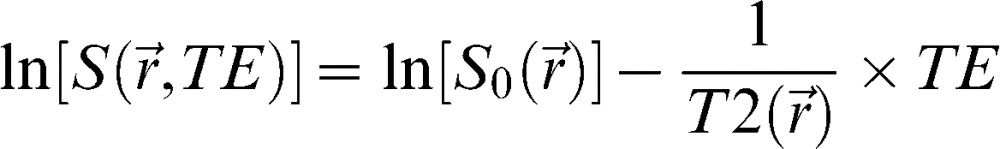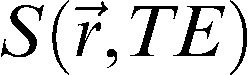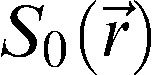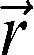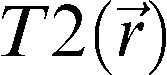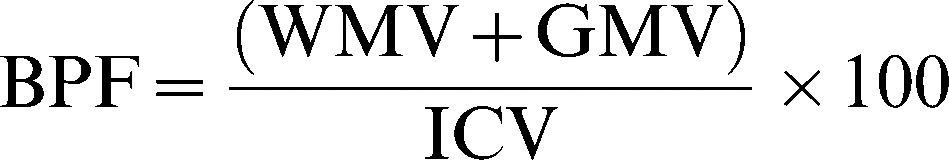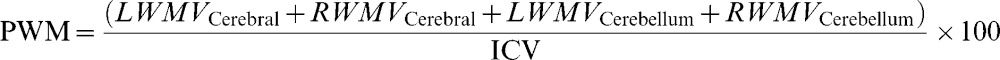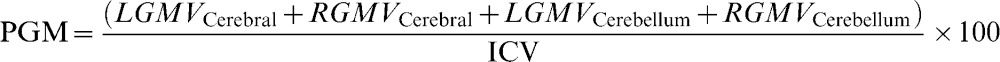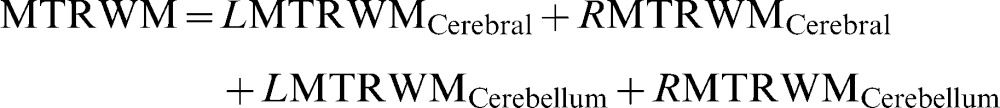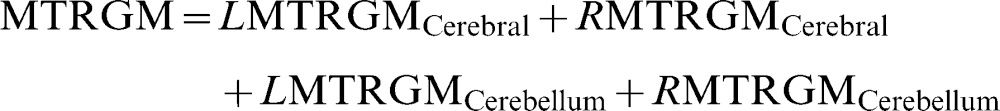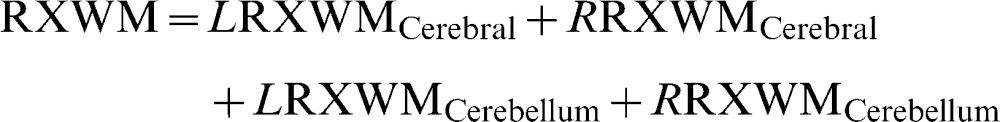To understand the relationships between brain structures and function (behavior and cognition) in healthy aging.
METHODThe study group was composed of 56 healthy elderly subjects who underwent neuropsychological assessment and quantitative magnetic resonance imaging. Cluster analysis classified the cohort into two groups, one (cluster 1) in which the magnetic resonance imaging metrics were more preserved (mean age: 66.4 years) and another (cluster 2) with less preserved markers of healthy brain tissue (mean age: 75.4 years).
RESULTSThe subjects in cluster 2 (older group) had worse indices of interference in the Stroop test compared with the subjects in cluster 1 (younger group). Therefore, a simple test such as the Stroop test could differentiate groups of younger and older subjects based on magnetic resonance imaging metrics.
CONCLUSIONThese results are in agreement with the inhibitory control hypotheses regarding cognitive aging and may also be important in the interpretation of studies with other clinical groups, such as patients with dementia and mild cognitive impairment.
The progressive degeneration of tissues, including brain parenchyma, is an expected event of aging even under normal physiological conditions. This fact has been documented not only in studies of brain volume, but also by other neuroimaging modalities sensitive to myelin impairment. Previous imaging reports showed volume decreases and diffusion facilitation related to aging. The loss of white matter (WM) integrity and gray matter (GM) volume is found mostly in the frontal lobe (1–3). The hippocampus also shows structural changes associated with aging, but they are less severe than those found in Alzheimer's disease and are not associated with the involvement of the area around the entorhinal cortex (1,4).
The prefrontal cortices, therefore, are most affected by aging compared with other neocortical regions, followed by volume loss in the temporal, parietal, and occipital lobes (5). Little research exists regarding subcortical structures in aging, but they appear to change little with aging. Additionally, it appears that the greatest shrinkage across the lifespan occurs mostly in the caudate and cerebellum (6).
Given this framework, age-related brain changes may be expected to correlate with cognitive dysfunction, which can be encountered in Alzheimer's disease (AD). Positive correlations have been found between AD and memory, executive functions, and frontal and temporal regions, whereas negative correlations have been described in elderly controls between executive functions, memory processes, and frontal regions (7). This result could be explained by the continual loss of cortical thickness and brain volume, including the “pruning” and neuronal reorganization inherent in human development, which results in the selection of the most capable neurons, leading elderly people with smaller brain volumes to exhibit performances that are higher than expected for their normative group. Other authors have reviewed the research in the past years and still encountered contradictory findings regarding the evidence that age-related changes in the volume of specific brain structures have direct consequences for cognitive function. Therefore, the relationships between structure (brain) and function (behavior and cognition) in healthy aging remain controversial.
Another area of interest is the age at which the greatest loss in cognition occurs. There is no consensus regarding whether it begins after age 70, after age 80, or even between these ages (8–11). Because of this lack of consensus, another of our objectives was to compare MRI markers of neuropsychological performance in a group of younger healthy elderly (12) with those in an older healthy elderly group (13) to understand the differences between these groups.
Regarding brain structure, the MRI analysis included volumetric quantitative techniques; relaxometry (quantitative T2), which is a marker of the presence of changes in the concentration of intra- and extracellular water in tissues; and evaluation of the magnetization transfer ratio (MTR), which is used to infer demyelination and axonal damage. In the neuropsychological assessment, techniques and tests that were sensitive to disorders associated with aging were applied and were translated into Portuguese.
Therefore, the objective of this study was to compare structural changes (both global and lobar gyri changes) on MRI with patterns of functional performance on neuropsychological tests to identify a connection between structure and function in the spectrum of normal aging.
PATIENTS AND METHODSStudy designThis was a prospective case-control study with normal elderly subjects submitted to an extensive medical evaluation to exclude both dementia and mild cognitive impairment (MCI). Both subgroups were studied with an extensive MRI protocol that included regular sequences and quantitative measurements of the cortical surface segmentation, brain parenchymal fraction (BPF), white matter (WM) and gray matter (GM) volume, magnetization transfer ratio (MTR), and quantitative T2 (qT2) of WM and GM. We extracted the values from maps of each group, and changes were evaluated based on these physical characteristics. The patients, based on their MRI characteristics, were divided into two subsets of individuals that exhibited higher (cluster 1) and lower (cluster 2) performance.
ParticipantsThe study group consisted of 56 elderly subjects, aged 58 to 83 years (mean±SD: 68.2±6.22 years), who had completed 1 to 15 years of schooling (7.4±4.37 years). All subjects were diagnosed as healthy, with the exclusion of any abnormality that may interfere with cognitive and cerebral functioning. The study was approved by the Research Ethics Committee, and all subjects provided written informed consent to participate in the study.
ProceduresOne expert geriatrician evaluated all participants to exclude medical conditions that may interfere with cognitive and cerebral functioning, such as diseases of the central nervous system that compromised cognitive function; sensorineural deficits (meaningful hearing loss, visual or color recognition deficit) or sensorimotor incapacitation that would impair the execution of the proposed tests; psychiatric disorder; history of chronic alcoholism (>3 drinks per day); clinical delirium; oxygen-dependent pulmonary conditions (partial pressure of oxygen less than 60 mmHg); pulmonary diseases or severe refractory asthma; endocrine-metabolic and nutritional conditions determined by laboratory tests, such as the venereal disease research laboratory (VDRL), calcium, fasting blood glucose, HMG, and TSH tests (i.e., diabetes mellitus, hypo/hyperthyroidism, hypercalcemia, growth hormone deficiency, conditions of hypo- or hypercortisolism, vitamin B12 or folic acid and niacin deficiency, and anemia with hemoglobin levels below 10 mg/dl); and cardiovascular disorders, including a diagnosis of severe or end-stage heart disease, a history of acute myocardial infarction or documented coronary disease, moderate to severe hypertension, and advanced atherosclerosis. Additionally, participants taking any of the following medications were also excluded: neuroleptics, tricyclic antidepressants, anticonvulsants, methyldopa, clonidine or similar drugs, corticoids (>5 mg of prednisone or equivalent), and benzodiazepines (for less than 6 months or at a dose of more than 10 mg of diazepam per day, or equivalent).
The instruments used to test study-eligible patients included the Brazilian version of the MINI International Neuropsychiatric Interview, which is a short, structured diagnostic interview used to diagnose psychiatric conditions, such as mood disorders and psychosis (14), and the Brazilian version of the Mini Mental State Exam (Folstein et al., 1975), adopting the criteria of 2 standard deviations above the mean for normal seniors with the same education level of this study group for exclusion of the diagnosis of dementia (3). The participants had to have scored 26-30 points on the Mini- Mental State Exam and passed the screening procedure in the short structured diagnostic interview (Mini International Neuropsychiatric Interview - Brazilian version). In addition, the subjects underwent activities of daily living assessment using the Katz scale, which must show preserved indices (scores: 0), i.e., independent activities of daily living scale (15) and Clinical Dementia Rating (CDR) scale, which includes indices of 0 and 0.5 (16). After this screening procedure, the participants proceeded to the MRI scan and neuropsychological assessment.
MRI protocolMRI images were acquired in a 1.5-Tesla MR machine (Magnetom Vision; Siemens, Erlangen, Germany). The image protocol included two identical axial gradient-echo sequences (TR = 34 ms; TE = 11 ms; slice thickness = 5 mm; and number of slices = 34), with and without a magnetization transfer pulse, used to generate the MTR map; one axial SE multi-echo sequence (TR = 3000 ms; TE = 22/60/120 ms; and 20 slices, 5-mm thickness), used to generate the T2 relaxometry map; and one sagittal volumetric 3DT1 MPRAGE sequence (TR = 9.7 ms; TE = 4 ms, and 160 slices 1-mm thick), used for volumetry and tissue classification.
MRI analysisSegmentation of regionsThe automated procedures for obtaining volumetric measurements of the different brain structures were described by Fischl et al. (17). This procedure was accomplished using the Freesurfer package version 4.5 (Martinos Center for Biomedical Imaging, Charlestown, Massachusetts, USA). This software classifies each voxel with a neuroanatomical label based on probabilistic information automatically estimated from a manually labeled training set. Initially, an optimal linear transform was computed to maximize the likelihood of the input image based on an atlas constructed from manually labeled images. Next, a nonlinear transform was initialized with the transformation of the previous step, and the image was deformed until a better match of the atlas was obtained. Finally, a Bayesian segmentation procedure was applied, and the maximum a posteriori estimate of the labeling was computed. The technique has been previously shown to be comparable in accuracy to manual labeling (17). The Freesurfer system provides the volumes of the classified structures and cortical thickness values.
MTR mapThe MTR map is a percent-difference image calculated voxel-by-voxel from one pair of identical sequences, except for the presence or absence of the MT pulse, using the following formula (18):
where (MO) is the signal intensity of the pixel with no MT pulse, Ms is the signal intensity of the pixel with the MT pulse, and MTR is the magnetization transfer ratio. We used the Mincmath tool (19) to construct the MTR map for each exam.Relaxometry mapA natural logarithm (ln) was applied in the multi-echo images. A linear equation results from this procedure:
where and represent the pixel intensity at the position in the image obtained at the TE echo time and “ideal” 0 echo time, respectively. Therefore, a linear least squares method can be used to estimate the T2 pixel value () from the obtained TE images. The in-house script “Relaxon” was implemented for this purpose using MINC tools.Estimation of global measures and cluster analysisAfter the segmentation, the global measures were computed. The following parameters were calculated:
Brain parenchyma fraction (%)where WMV is the white matter volume, GMV is the gray matter volume, and ICV is the intracranial volume.Percentage of white matter (%)where LWMV is the white matter volume of the left hemisphere, RWMV is the white matter volume of the right hemisphere, and ICV is the intracranial volume.Percentage of gray matter (%)where LGMV is the gray matter volume of the left hemisphere, RGMV is the gray matter volume of the right hemisphere, and ICV is the intracranial volume.MTR of white matter (%):where LMTRWM is the white matter MTR of the left hemisphere and RMTRWM is the white matter MTR of the right hemisphere.MTR of Gray matter (%):where LMTRGM is the white matter MTR of the left hemisphere and RMTRGM is the white matter MTR of the right hemisphere.Relaxometry of white matter (ms):where LRXWM is the white matter relaxometry of the left hemisphere and RRXWM is the white matter relaxometry of the right hemisphere.Relaxometry of gray matter (ms):where LRXWM is the white matter relaxometry of the left hemisphere and RRXWM is the white matter relaxometry of the right hemisphere.The patients, based on their MRI characteristics, were divided using k-means cluster analyses implemented in Minitab software, and two groups were formed: cluster 1, which had the best results with respect to quantitative MRI measures (high volumes and MTRs), and cluster 2, which had the worst results (low volumes and MTR values and high T2 values). The k-means cluster analysis is used to cluster observations into groups when the groups are initially unknown. This procedure uses non-hierarchical clustering of observations.
Cluster analysis and comparisonUsing the MTR, relaxometry, volumes of cortical and subcortical structures, white matter areas, and cortical thickness values, we compared the clusters with the goal of identifying the specific localization of lesions. For this comparison, we used the Kolmogorov-Smirnov test to analyze the normality of the samples and the two-tailed t-test for independent samples.
Characterization of neuropsychological indexesHealthy individuals were submitted to the neuropsychological evaluation using the Mattis Dementia Rating Scale (MDRS) (20), Stroop Test (21,22), Verbal Fluency test (23), Wisconsin Card Sorting Test (WCST) (24), Rey Complex Figure test (25), Vocabulary – Wais – III (26), Logical Memory (WMS-R) test (27), Visual Reproduction (WMS-R) (27) and Rey Auditory-Verbal Learning Test (RAVLT) (28), conducted by a neuropsychologist with experience in cognitive assessment of elderly individuals. Other demographic variables were collected by informal questionnaires with the patient such as schooling, occupation, socioeconomic level and handedness.
Data analysisThe performance of each participant during the neuropsychological evaluation was standardized in z-scores according to the mean and standard deviation of this study group and the signs were inverted for the measures for which a higher score indicated a poorer performance, as was the case for the measures of processing time.
These neuropsychological results were divided according to the quantitative MRI measures, and a comparison between the groups was performed by the Mann-Whitney nonparametric test of the SPSS 13.0 for Windows including the variables age and schooling.
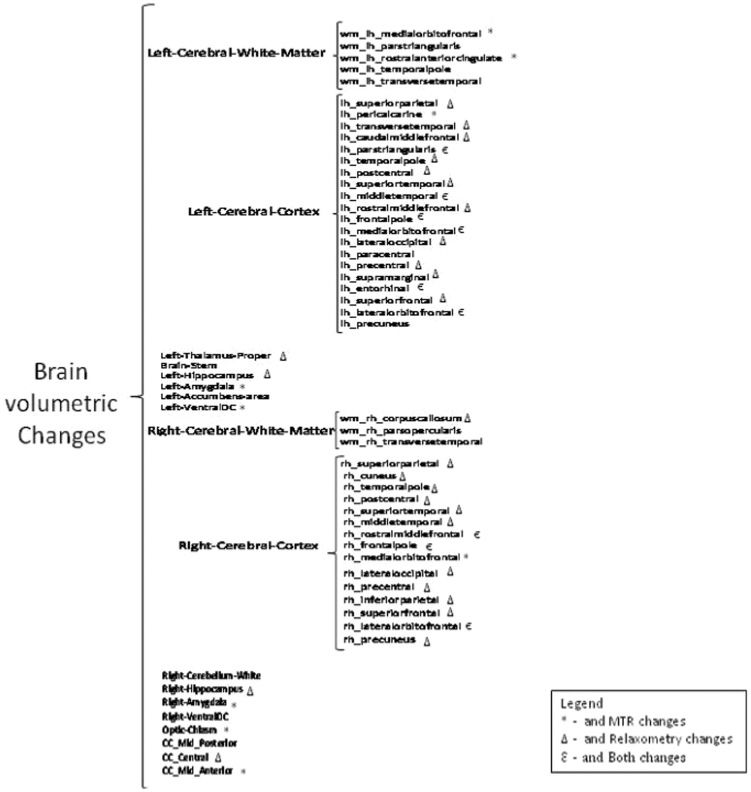
RESULTSThe cluster analysis identified one group in which the brain MRI markers were more preserved and another in which the brain MRIs showed less preserved markers. The cluster 1 had the best results with respect to quantitative MRI measures (high volumes and MTRs) and cluster 2 had the worst results (low volumes and MTR values and high T2 values). With regard to volume, cluster 1 had higher significant values than the second cluster at global (brain parenchyma fraction (p = 0.02) and local levels (left brain white matter (p = 0.02), left cerebral cortex (p = 0.03), right brain white matter (p = 0.004), and right cerebral cortex (p = 0.02) (Figure 1). The cortical regions had a greater number of distinct regions compared with WM and subcortical structures. There was also an increased involvement of the left cerebral cortex compared with the right cerebral cortex, for both gray and white matter. The frontal and temporal lobes were more affected than the other regions in the two groups.
The MTR showed predominant changes in WM and subcortical structures, with more conserved values in cluster 1 (p<0.05) (Figure 1). The qT2 showed more extensive abnormalities than MTR, especially in the right cerebral cortex (Figure 1). The MTR and qT2 demonstrated more preserved indices for cluster 1 in the pars triangularis, medial temporal regions (including entorhinal regions), the pre-motor frontal lobe of the left cerebral cortex, and the right cortical regions of the frontal lobe. Volume analysis of subcortical structures revealed more preserved values for cluster 1 in the hippocampus, amygdala, and ventral diencephalon bilaterally, as well as in the left thalamus, brain stem, left accumbens area, and right cerebellum. The middle posterior corpus callosum, anterior middle corpus callosum, and central corpus callosum (Figure 1) also demonstrated differences between groups. The differences in magnetization transfer between the clusters were significant in the left ventral diencephalon, optic chiasm, mid-anterior corpus callosum, and bilaterally in the amygdale. The qT2 revealed better indices for cluster 1 in the left thalamus proper, left and right hippocampus, and central corpus callosum.
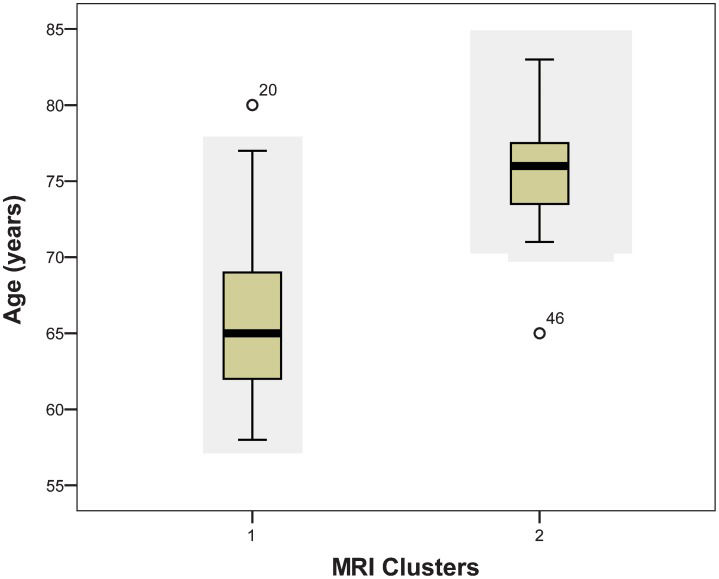
The participants in cluster 1 had a mean age of 66.4±5.2 years and 7.3±4.2 years of schooling, while those in cluster 2 had a mean age of 75.4±4.8 years and 7.8±5.2 years of schooling. Occupations varied in clusters 1 and 2, with half of the participants being retired (51-54%), 26-18% being housewives, and 22-27% being employed. The right-hand preference was prevalent for clusters 1 and 2. In both clusters, 55-54% of the participants were of low socioeconomic status, while 44-45% were of middle socioeconomic status. There were no significant differences (p<0.05) between the clusters with regard to education, occupation, handedness, and socioeconomic level, but significant differences in age were found.
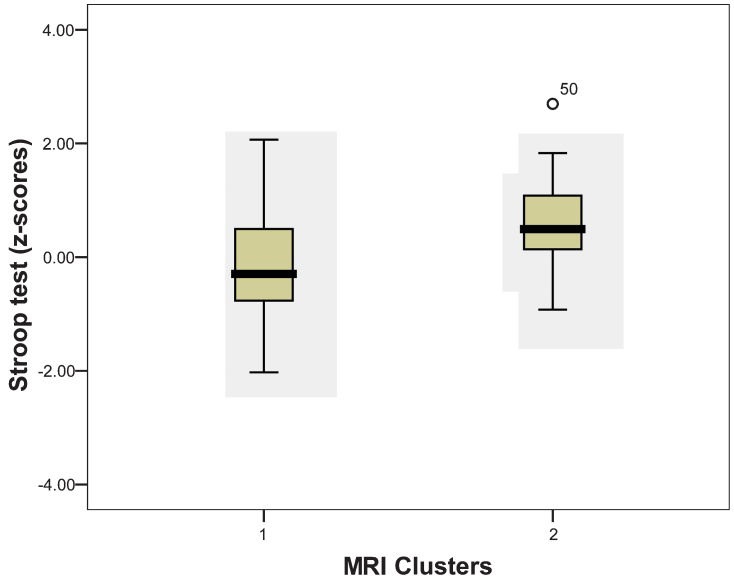
Due to the differences in neuropsychological performance between cluster 1 (mean age of 66.4 years) and cluster 2 (mean age of 75.4 years), we encountered significant differences in the interference indices of the Stroop test (p = 0.024) (Figure 2). In addition, cluster 1 had better weighted scores (z-score: +0.142) than cluster 2 (z-score: -0.650). There were also significant differences in age between the clusters (p<0.0001, Figure 3, whereas no significant difference was noted in years of schooling (p = 0.536). Therefore, the older group, which had worse indices of interference in the Stroop test, also had inferior results in MRI than the younger group.
DISCUSSIONControversy remains regarding the relationship between brain volumes and cognition in healthy elderly, yet changes in the aging brain structure are shown by MRI and described as an increase in size of the cerebral ventricles and reduction in volume of the cerebral cortex, hippocampus, and parahippocampal gyrus related to aging (29). However, cognitive declines in aging involve mechanisms other than those that are purely structural, such as alterations in the production of neurotransmitters (dopaminergic explanation), dysfunction of some cognitive domains (inhibitory control hypothesis, hypothesis of slow mental processing, and frontal hypothesis), and sensory losses, among others. These theories are not mutually exclusive, and a theory that unifies and explains the interrelated mechanisms of cognitive aging in these different levels is still missing (1).
Previous studies in this area seem to consider only one side of the equation between brain and behavior, without explaining their relationships; therefore, the present study is important because it compared MRI results with neuropsychological performance in two groups of healthy elderly, one in the sixth decade of life and another in the seventh decade of life. This study began with the analyses of biological markers associated with MRI findings in two clusters, namely cluster 1 (mean 66.4 years), consisting of participants who had less volumetric change in MTR and qT2, and cluster 2 (mean 75.4 years), consisting of participants who had global atrophy, a low MTR, and a prolonged T2 time. Such findings are in accordance with studies that indicate a more rapid decline in cognition after 70 years of age (8,9). These results did not show differences with respect to other variables such as educational level, occupation, socioeconomic level, and handedness, which could explain such discrepancies.
The MRI results showed that cortical regions were more affected than white matter and subcortical structures in the older cluster. Other studies demonstrated that cortical gray matter atrophy predicted cognitive decline regardless of whether lacunes were present; neither lacunes nor white matter hyperintensity independently predicted decline (30). Although many histological studies have demonstrated that white matter changes are more prominent than cortical changes with aging (31–37), other authors indicated a greater loss of gray matter in mild cognitive impairment (MCI), with a greater risk of conversion to dementia than in stable MCI (38). Another study using a cross-sectional, population-based VBM also revealed an accelerated gray matter loss exclusively in men, involving the temporal neocortex, prefrontal neocortex, and medial temporal regions (39). Even though our study included more women than men, it did not reveal differences related to gender.
Subcortical structures were less affected by age than cortical structures, but significant differences (p<0.05) were found in the volume of the left thalamus proper, brain stem, left acumens area, and right cerebellum white matter, as well as bilaterally in the hippocampus, amygdale, and ventral diencephalon. These findings are comparable to those of other studies indicating that the greatest shrinkage across the lifespan occurs in the caudate, cerebellum, hippocampus, and prefrontal areas (6). Previous studies also argued that subcortical structures, such as the striatum, have not been extensively studied in aging, and similarly little is known about changes in the volume of the cerebellum (5,40,41). According to the present study, the subcortical structures do not seem to change significantly with age, as we verified more involvement of the cortical regions.
The MTR provides an indication of the quantity of protons bound to macromolecules in a given tissue, and our study revealed WM impairment in addition to volume loss. The MTR is reduced in many pathological processes, particularly those associated with demyelination and axonal damage (42). Decreases in the MTR have been reported in experimental models of Wallerian degeneration after optic nerve lesions (43).
Clinical studies have reported that MTR measurements show major reductions in processes related to demyelination, axonal loss, and tissue destruction (44). The MTR has been extensively used for the assessment of central nervous system WM lesions in different diseases, especially in MS, in which the MTR is used to predict disease progression (45–48). Although brain atrophy may be associated with the MTR decrease, pathological correlations have demonstrated that the main cause of the MTR decrease is demyelination. In addition, in normal-appearing WM, the MTR decrease correlates with axonal attenuation. Therefore, we speculate that the MTR decrease in our cohort may be explained by a combination of neuronal and axonal damage and demyelination related to aging.
The qT2 showed abnormalities in cortical structures related to the right cerebral cortex, as well as the MTR. Both of these MRI metrics revealed changes in cortical structures that were most severe in the second cluster in the left cortex associated with the temporal-frontal cortex and right cerebral cortex of the frontal lobe.
Therefore, our data demonstrated that the frontal and temporal lobes were preferentially affected (Figure 1). Many studies also concluded that the prefrontal cortices were the most affected by aging compared with other neocortical regions, followed by losses in parietal, temporal, and occipital cortices (1,5,8).
As already cited, the cortical areas were more affected than the other areas, and the relationship between gray matter loss and executive functions probably indicates processes that affect the frontal cortex by direct neuronal loss, loss of neocortical association ascending pathways, or damage to fronto-subcortical circuits (49). Executive functioning was impaired with the increase in white matter hyperintensities and the loss in volume of the prefrontal cortex (50). The analysis of the MTR in this study indicated lower values in the anterior regions of the brain in cluster 2, suggesting that this group of older elderly individuals with less preserved brain volume and worse neuropsychological scores possesses smaller areas of myelination than cluster 1. Thus, decreased myelination of nerve fibers appears to influence the biological and neuropsychological measures of aging (inhibitory control) even in a healthy aging group.
The decrease in MTR and the increase in relaxation time of the brain MRI is a sign of healthy tissue loss that may be observed more in the left cerebral hemisphere, mainly in the temporal and frontal areas, whereas these occurrences were confined to the frontal lobe in the right hemisphere cortical areas. This finding suggests greater tissue integrity loss in anterior areas of the brain, especially in the frontal cortex.
The vulnerability of the frontal lobes to cognitive aging is the basis of one of the most accepted theories of cognitive aging known as the Frontal Lobe Hypothesis of Aging. This hypothesis postulates that the decline in frontal efficiency can account for many of the cognitive deficits associated with cognitive aging, such as impared executive functions (1).
The Stroop test, Color and Trail-Making Test, Word Fluency tests, and Wisconsin Card Sorting Test (WCST) were administered to evaluate executive functions. The Stroop test (interference index) was the only test that indicated differences between clusters 1 and 2, with worse MRI measures found in the older elderly (cluster 2) compared with the younger elderly cluster (cluster 1). The effect of age on the Stroop interference paradigm could be attributable to the slowness in mental processing time; however, this slowness would lead to a decrease in the reading speed for incongruent words, leading to the Stroop interference effect (41). In the current study, the rate of interference was measured by dividing the time (in seconds) necessary to name colored dots by the time required to name the colors of words written with the names of different colors.
Changes in inhibitory control constitute one of the hypotheses about cognitive aging that may relate it to a healthy process of human development. However, other authors pointed out that errors in the color incongruent trials, in a computerized version of the Stroop test, along with differences between congruent and incongruent trials (interference index), were the strongest predictors of conversion to Alzheimer's disease (AD) (51). Accordingly, deficits in the Stroop test could be an incipient symptom of the development of pathological states that are not exclusively related to cognitive aging and deserve other follow-up.
According to our results, signs of neuronal loss were more frequent in the frontal lobe in both clusters but could also be observed in the left temporal lobe. Other authors showed greater gray matter loss in the hippocampus and hippocampal structures and less loss in the posterior cingulate cortex, inferior temporal gyrus and middle fusiform gyrus, precuneus, tempoparietal junction, and frontal cortex in the pre-clinical stages of dementia, which diverges from the results in our study group and seems to place them at a higher risk for dementia (38).
In general, different authors have linked memory decline with hippocampal volume loss in elderly individuals (5,30,40,49,50). However, our results did not reveal such a correlation, probably because this series was restricted to healthy elderly participants, which may not be representative of the normal aging group as a whole. Moreover, it was not possible to compare these results with population normative data that could explain the lack of significant differences between groups in measures of memory. Indeed, it must be considered that the assessment of cognitive decline was beyond the scope of this work because it was not a longitudinal study.
In conclusion, our data allow the inference that the heterogeneity of normal aging can be expressed functionally and structurally, which may explain the overlap between normal and pathological groups in studies comparing mild cognitive impairment and early dementia. From a functional point of view, these results are in accordance with the inhibitory control hypotheses regarding cognitive aging. Investigations of the normal spectrum of aging are essential for the study of the pre-clinical stages of all degenerative processes associated with aging and can highlight some biological and neuropsychological markers of incipient cases of dementia.
AUTHOR CONTRIBUTIONSFoss MP recruited patients, performed neuropsychological testing, conceived and conducted the analyses of neuropsychological assessment data, performed statistical analysis, and served as the main author of the article. Diniz PR developed the parameters, analyzed the magnetic resonance imaging (MRI) data, performed the statistical analysis, generated the MRI data, and revised the manuscript. Formighieri PF examined the patients for inclusion in the study and reviewed the manuscript. Salmon CE created the software to analyze the RMI, contributed to the article, performed MRI data analysis, and revised the manuscript. Speciali JG served as the post-graduate supervisor of the project and revised the manuscript. Santos AC served as the second post-graduate supervisor of the project, analyzed the MRI data, and revised the manuscript.
No potential conflict of interest was reported.