This study focused on circulating levels of vascular endothelial growth factor in patients with prostate cancer compared to a normal population.
METHODS:We analyzed 26 normal individuals and 80 patients with prostate cancer. Blood was drawn from all subjects, and plasma was extracted to determine the concentration of vascular endothelial growth factor using a quantitative immunoassay technique (ELISA—enzyme-linked immunosorbent assay).
RESULTS:The median plasma level of vascular endothelial growth factor was significantly elevated in patients with metastatic disease compared to patients with localized disease and with healthy controls. Patients with serum prostate-specific antigen > 20 ng/mL had significantly higher levels of plasma vascular endothelial growth factor than patients with serum prostate-specific antigen < 20 ng/mL. There was a trend for patients with a Gleason score of 8 to 10 to have higher levels of plasma vascular endothelial growth factor when compared to patients with lower Gleason scores. No relationship was found between plasma vascular endothelial growth factor and clinical staging, or between plasma vascular endothelial growth factor and prostate volume, in patients with localized prostate cancer.
CONCLUSION:This study indicates that patients with metastatic prostate cancer have higher plasma vascular endothelial growth factor levels than patients with localized disease or in healthy controls.
Analisar os níveis circulantes do fator de crescimento do endotélio vascular em pacientes com câncer prostático comparados com uma população de indivíduos eutróficos.
MÉTODOS:Vinte e seis indivíduos eutróficos e oitenta pacientes com câncer de próstata foram analisados nesse estudo. A coleta sangüínea foi realizada da mesma maneira em todos os pacientes e o plasma foi extraído para a determinação dos níveis do fator de crescimento do endotélio vascular, utilizando-se o método quantitativo ELISA (enzyme-linked immunosorbent assay).
RESULTADOS:Os níveis de fator de crescimento do endotélio vascular plasmático encontraram-se significativamente elevados nos pacientes com doença metastática quando comparados com pacientes com doença localizada e com indivíduos sadios. Pacientes com PSA sérico maior que 20 ng/ml apresentaram níveis maiores de fator de crescimento do endotélio vascular plasmático quando comparados com pacientes com PSA menor que 20 ng/ml. Houve uma tendência dos pacientes com escore de Gleason de 8 a 10 apresentarem níveis maiores do fator de crescimento do endotélio vascular plasmático em relação a pacientes com escores de Gleason menores que 8. Não houve relação entre fator de crescimento do endotélio vascular plasmático e estado clínico, ou entre fator de crescimento do endotélio vascular e volume prostático em pacientes com câncer de próstata localizado.
CONCLUSÃO:Os dados indicam que pacientes com câncer de próstata metastático apresentam níveis significativamente mais elevados de fator de crescimento do endotélio vascular plasmático quando comparados com pacientes com câncer localizado e com indivíduos normais.
The incidence of prostate cancer in Brazil is high. There were 32,240 new cases and 8,230 deaths due to prostate cancer in Brazil according to the most recent data from the Instituto Nacional do Câncer (INCA) in 2003.1 Angiogenesis is defined as the development of new blood vessels for tissue formation. The development of a vascular supply is essential for organ formation and regeneration, tissue differentiation during embriogenesis, wound healing, reproductive functions in adults, and tumor tissue formation.2 Angiogenesis, important for tumor formation, can be measured using microvessel density and circulating levels of related growth factors such as vascular endothelial growth factor (VEGF). Vascular endothelial growth factor plays an important role in angiogenesis regulation, and its role in human tumor formation is well established. The overexpression of VEGF in human tumors is related to increased vessel density and poor prognosis.2 Expression of VEGF in normal prostate tissue was first described in 1995 by Brown et al.3 After this study, several groups reported the expression of VEGF in prostatic tissues, demonstrating that VEGF is overexpressed in tumors and in poorly differentiated tissues when compared to normal tissues, proving the importance of VEGF in the pathogenesis of prostate cancer.4–6
The measurement of circulating levels of VEGF was initially described by Yamamoto et al,7 who reported that serum VEGF levels were higher in cancer patients when compared to normal individuals. Subsequently, other articles analyzing circulating levels of VEGF in many types of cancer have been published.8 Serum levels of VEGF have been correlated with stage of disease in colorectal cancer. In patients with lung cancer, serum levels of VEGF have been correlated with poor clinical outcome (survival and treatment response). Patients with liver cancer, particularly those with metastatic disease, had higher circulating VEGF levels when compared to normal individuals. A strong correlation between circulating VEGF levels and advanced or metastatic breast cancer has been reported. There is controversy among different studies analyzing circulating VEGF levels in ovarian cancer.
Recently, several groups have reported that the analysis of circulating VEGF levels in serum is problematic because VEGF is present in platelets and is released during the clotting process. Consequently, the quantitative measurement of VEGF by ELISA can be directly affected by number of platelets present in the serum sample. Most measurements of circulating VEGF levels in cancer patients published in the literature have been of serum VEGF levels. Banks et al claims that serum VEGF measurements are “totally unsuitable” and recommended the use of citrate or ethylenediaminetetraacetic acid (EDTA) plasma for VEGF quantitation in blood.9
The purpose of this study was to quantify plasma VEGF in patients with prostate cancer and in normal individuals, and to correlate plasma VEGF levels with clinical stage, serum PSA, histological grade, and prostate volume in patients with prostate cancer.
METHODSSubjectsA total of 80 patients with prostate cancer (from a single institution, diagnosed in 1998) and 26 cancer-free individuals (control group) were retrospectively analyzed. Control subjects older than 50 years presented with a normal digital rectal examination and normal prostate-specific antigen (PSA) levels (values between 0 and 4 ng/mL using the Hybritech® PSA test; Hybritech, Fullerton, CA). All clinical information from the 80 patients with prostate cancer was extracted from clinical records. Fifty-four patients were considered to have localized prostate cancer according to clinical parameters (digital rectal examination, PSA, and bone scan). Twenty-six patients were considered to have metastatic disease according to a positive bone scan or histologic confirmation of cancer metastasis to the pelvic lymph node after node dissection. Patients with metastatic disease had not been hormonally manipulated before blood withdrawal. For clinical classification of patients with localized disease, the TNM classification system was adopted (1997). The clinical classification of patients with localized disease was performed by different urologists. We grouped them into T1c, T2, and T3 groups to avoid data dispersion. To analyze the correlation between VEGF and PSA, a PSA value of 20 ng/mL was adopted as a cutoff, since patients with PSA >20 ng/mL have a much higher incidence of metastatic disease.10 For the analysis of histological grade, we divided cancer patients into 3 groups: patients with poorly differentiated tumors (Gleason score, 8, 9, and 10), patients with moderately differentiated tumors (Gleason score, 7) and well differentiated tumors (Gleason score, 5 and 6); because of their distinct behavior.11 Prostate volume was estimated by transrectal ultrasound in 51 out of 54 patients with localized disease.
Vascular endothelial growth factor (VEGF) measurementsPeripheral blood samples were drawn from the arm for all subjects, placed immediately in sterile tubes containing 0.057 mL of 15% EDTA, taken immediately to the laboratory for processing of plasma (centrifugation at 1000 x g), and stored in a liquid nitrogen freezer at -70°C. One freeze-thaw cycle was used for all samples. The VEGF concentrations were determined using an ELISA method (Quantikine, R&D Systems, Minneapolis, Minn), according to the manufacturer’s protocol. Values were calculated and converted to picograms per milliliter (pg/mL).
Statistical analysisThe Pearson correlation coefficient was calculated as a measure of linear association between continuous variables, including age, serum PSA, prostate volume, and plasma VEGF. The Kolmogorov-Smirnov test revealed significant departures from normality for plasma VEGF levels. Consequently, median VEGF levels were compared among the 3 groups of patients (controls, localized disease, and metastatic disease) using the nonparametric Kruskal-Wallis 1-way layout procedure, followed by the Mann-Whitney U test. Vascular endothelial growth factor levels were also compared among Gleason score and clinical stage subgroups with the same approach. A Bonferroni correction was used to adjust the type 1 error rate for multiple comparisons, such that 2-tailed P < 0.017 was considered significant. Data analysis was performed using Statistical Analysis System version 6.12 statistical software.
RESULTSCorrelation between plasma VEGF levels and ageThe median age of patients in the control group was 48.5 years (from 32 to 69 years), and 66 years in the prostate cancer group (45 to 84 years). The median age of the control group was significantly lower than the median age of the cancer group (P < 0.05). However, we found no relationship between age and plasma VEGF in patients with localized disease, metastatic disease, or controls (r = 0.10; P = 0.29) (Figure 1).
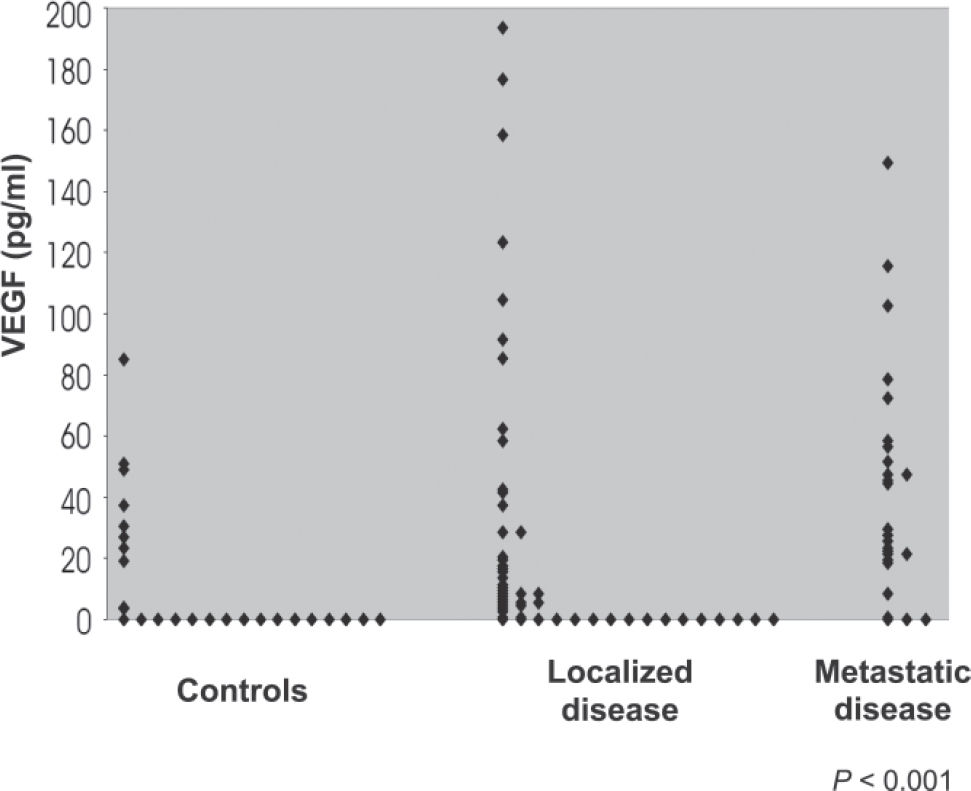
Comparison of plasma VEGF levels in patients with localized disease, metastatic disease, and control groupThe median levels of VEGF in the 3 groups (controls, n = 26; localized disease, n = 54; and metastatic disease, n = 26) were compared. The Kruskal-Wallis test showed significant differences among the 3 groups (P < 0.001). The Mann-Whitney U test reveled a significant difference between controls and patients with metastatic disease, and between patients with localized and metastatic disease (P <0.001 and P <0.003, respectively). Table 1 and Figure 2 show the distribution of VEGF values among the 3 groups.
Plasma vascular endothelial growth factor (VEGF) concentrations in the 3 groups analyzed
| Group | n | Median VEGF (pg/mL) | Mean VEGF (pg/mL) |
|---|---|---|---|
| Controls | 26 | 0 (0-24)* | 12.7 |
| Localized disease | 54 | 7.0 (0-26.5) | 26.7 |
| Metastatic disease | 26 | 28.5 (19.3-57) | 41.9 |
P < 0.001; *Number in parentheses = interquartile range
The Gleason scores obtained from prostate needle biopsy were analyzed for an association with plasma VEGF levels. Six patients were excluded from the study because of lack of data. One patient with Gleason 2 + 2 was excluded. Therefore, 73 patients were analyzed. There were no differences in plasma VEGF levels among the groups based on the Kruskal-Wallis test (P = 0.18). However, there was a trend for VEGF to be higher among patients with Gleason score 8 to 10 (Table 2).
Correlation between plasma VEGF levels and serum PSA in patients with prostate cancerWe did not observe a linear correlation between serum PSA and plasma VEGF levels (r = 0.14; P = 0.22). However, patients with PSA levels greater than 20 ng/mL had significantly higher plasma VEGF values than patients with PSA levels less than 20 ng/mL (Table 3). Two patients were excluded for not having PSA levels determined at the time of the analysis.
Correlation between clinical stage and plasma VEGF in patients with localized diseaseRegarding the 54 patients with localized prostate cancer, VEGF levels were not related to clinical stage (P = 0.54). Six patients were excluded from the analysis for not having clinical stage described in the clinical records. Table 4 shows plasma VEGF levels according to clinical stage.
Correlation between prostate volume and plasma VEGF levels in patients with localized prostate cancerNo correlation between plasma VEGF levels and prostate volume in patients with localized disease were found (Table 5). Three patients were excluded from the analysis because they did not have measurements of prostate volume when transrectal ultrasound was performed.
DISCUSSIONAngiogenesis, essential for tumor formation and the object of intense research, can be measured by microvessel density and by measurement of circulating levels of related growth factors, such as VEGF. Several studies suggest that the measurement of circulating levels of VEGF is a useful tool for analyzing prognosis and clinical outcome in many tumors. In the present study, plasma levels of VEGF was correlated with several parameters related to prostate cancer, such as age, serum PSA, histological grade, clinical stage, and prostate volume.
No correlation was found between plasma VEGF and age of the patients. In the selection of the control group a younger population sample was deliberately collected in order to avoid inclusion of patients with occult prostate cancer in this group. Accordingly, Yamamoto et al analyzed 184 normal individuals with ages varying from 21 to 59 years and found no correlation between age and circulating VEGF levels.7 Kumar et al also found no correlation between VEGF levels and age in a population of 136 individuals with ages varying from 20 to 80 years.12
In this study, plasma VEGF levels were significantly higher in patients with metastatic prostate cancer in comparison to patients with localized disease and healthy controls (P <0.001, Table 1). These findings are consistent with the study of Salven et al, which found elevated VEGF levels in patients with metastatic disease, although the fluid analyzed was serum in that study.13
Reported data regarding the measurement of circulating VEGF levels in patients with prostate cancer is scarce. Jones et al measured VEGF in the serum of 78 individuals (21 controls, 9 with benign prostate hyperplasia, 16 with localized prostate cancer, and 32 with metastatic prostate cancer) and found elevated VEGF levels only in patients with hormonal-refractory metastatic prostate cancer. 14 They found no differences in serum VEGF levels among controls, localized disease, and metastatic disease. They did not analyze VEGF levels in regard to clinical stage, Gleason score, or prostate volume. Their control group was also composed of younger individuals. In our study, patients with metastatic disease were not subdivided into hormone-refractory and hormone-sensitive groups. Therefore, the data cannot be compared with regard to this issue. Additionally, the 2 studies were not comparable because the fluid analyzed was different (plasma vs serum). Several studies in the literature analyzing circulating VEGF levels in patients with cancer, report high levels of this molecule in patients with advanced or metastatic cancer. 7,13 Such data are compatible with both the study of Jones et al and our study. The fact that Jones et al14 did not find differences between patients with localized and metastatic disease may reflect the small sample of their study (16 patients with localized and 23 patients with metastatic disease).
Another study with 197 patients with hormone-refractory prostate cancer reported plasma VEGF levels before and after treatment with suramin (patients in the protocol had already received hormones), and found an inverse correlation between plasma VEGF and survival. Yet, using a cutoff of 260 pg/mL, the plasma VEGF level was the strongest predictor of survival in a multivariate analysis, including other predictors such as serum PSA and alkaline phosphatase.15 The data suggests that plasma VEGF may be clinically relevant in patients with hormone-refractory prostate cancer in terms of prognosis. However, plasma VEGF may be more than a disease marker, also reflecting a specific phenotype of a cancer that produces VEGF. Prospective studies including tumor histological analysis should be conducted to clarify this hypothesis.
Recently, Kohli et al reported a prospective study comparing two groups of patients with advanced prostate cancer in order to determine whether there is correlation between plasma VEGF and tumor progression (from hormone-sensitive to hormone-refractory).16 The first group was composed of patients with stable PSA during hormonal treatment, and the second group had patients with progressive PSA levels during treatment. There was no difference between the two groups in regard to plasma VEGF levels. One could argue that the development of metastatic spots would depend on angiogenesis and, therefore, would be accompanied by higher levels of related growth factors (like VEGF) in patients with disease progression. Alternatively, the increase in serum PSA levels in patients with disease progression may exert an anti-angiogenic effect, lowering circulating VEGF. The PSA anti-angiogenic effect on VEGF occurs due to its proteolytic action on plasminogen, creating an angiostatin-like protein with an anti-angiogenic effect.
In this study, we found not only a relationship between VEGF and advanced disease, but also a relationship between VEGF and other clinical parameters associated with prostate cancer (PSA, clinical stage, and Gleason grade). Patients with metastatic disease had significantly higher VEGF levels than patients with localized disease and controls. Although we did not find significant differences between the control group and patients with localized disease, we observed a tendency of patients with localized disease to have higher VEGF levels than controls. The lack of statistical significance could be attributed to the small sample size; a larger series may or may not confirm this hypothesis. Yet, some patients with localized disease in this study had high VEGF levels. The reason for elevated plasma VEGF levels in patients with localized disease is unknown. Prospective study and long term follow-up of these patients should reveal whether patients with high plasma VEGF levels present worse prognoses than patients with low plasma VEGF levels. Even some patients in the control group were found to have high plasma VEGF, for unknown reasons. We should be aware that circulating VEGF may be elevated in patients with other, noncancerous disease.
We found no relationship between plasma VEGF and clinical stage in patients with localized disease. Two issues should be noted, ie, the limited sample size and the fact that different urologists performed the digital rectal examination and clinical staging.
There was no relationship between plasma VEGF levels and Gleason score; however, we observed a tendency of patients with high Gleason scores (8 to 10) to have higher VEGF levels than patients with low Gleason score (5-6 or 7). Prostate cancer patients with high serum PSA levels and high Gleason scores are more likely to have metastatic disease.
No linear correlation between plasma VEGF and serum PSA levels were observed. However, patients with PSA levels greater than 20 ng/mL had significantly higher VEGF values than patients with PSA levels less than 20 ng/mL. This suggests that plasma VEGF is elevated in patients with disseminated disease. When prostate cancer presents as an organ-confined disease, plasma VEGF levels may not be elevated, regardless of clinical stage, Gleason grade, and PSA values.
In our study, there was no correlation between prostate volume and VEGF levels in patients with localized disease. Kohli et al, analyzing patients with localized prostate cancer, found no differences between patients who had undergone radical prostatectomy and patients with the intact gland (before treatment) with regard to plasma VEGF levels; this suggests that the prostate gland does not influence VEGF levels.16 Although the results of both studies are the same, the data are not comparable, because the respective populations are totally different.
The results of our study suggest that plasma VEGF is not affected by prostate size. However, George et al compared plasma VEGF levels in patients before and after radical prostatectomy, and found a significant decrease in VEGF levels after surgery, suggesting that the prostate gland may be an important source of circulating VEGF.17
Additionally in this study, there was no correlation between plasma VEGF and other prognostic factors related to prostate cancer before radical prostatectomy (PSA, clinical stage, Gleason grade). However, Shariat et al measured plasma VEGF in 215 patients with localized prostate cancer before radical prostatectomy and found a correlation between plasma VEGF and prostate volume, Gleason grade obtained from prostate needle biopsy and definitive surgery, extra-prostatic disease, positive lymph nodes, and biochemical failure after surgery.18 The control group also had lower VEGF values than patients with localized prostate cancer. Nine patients with metastatic disease had higher VEGF values when compared to the group of 215 patients with localized disease.
Based on all available published papers, controversies and unanswered questions persist about the role of circulating VEGF in prostate cancer patients. The role of VEGF remains unknown, in part due to its low specificity. Prospective studies with long-term follow-up may determine whether circulating VEGF can be used as a prognostic marker in patients with localized and metastatic disease, or as a tool in selecting patients who will benefit from curative treatment. The value of VEGF in predicting lymph node disease remains uncertain. Yet, VEGF can be tested as a marker of response to treatment in patients with metastatic prostate cancer.
CONCLUSIONThis study indicates that patients with metastatic prostate cancer have significantly higher plasma VEGF levels than patients with localized disease or healthy controls.