MicroRNAs are noncoding RNA molecules involved in the development and progression of tumors. We have found that miRNA-100 is underexpressed in metastatic prostate cancer compared to localized disease. Conversely higher levels of miR-100 are related to biochemical recurrence after surgery. This suggests that miR-100 may be a context-dependent miRNA, acting as oncogene or tumor suppressor miRNA. Our aim is to demonstrate the role of miR-100 in the control of predicted target genes in prostate cancer cell lines.
METHODSCell lines DU145 and PC3 were transfected with miR-100, antimiR-100 and after 24 h and 48 h of exposure, qRT-PCR and western blot were performed for mTOR, FGFR3, THAP2, SMARCA5 and BAZ2A.
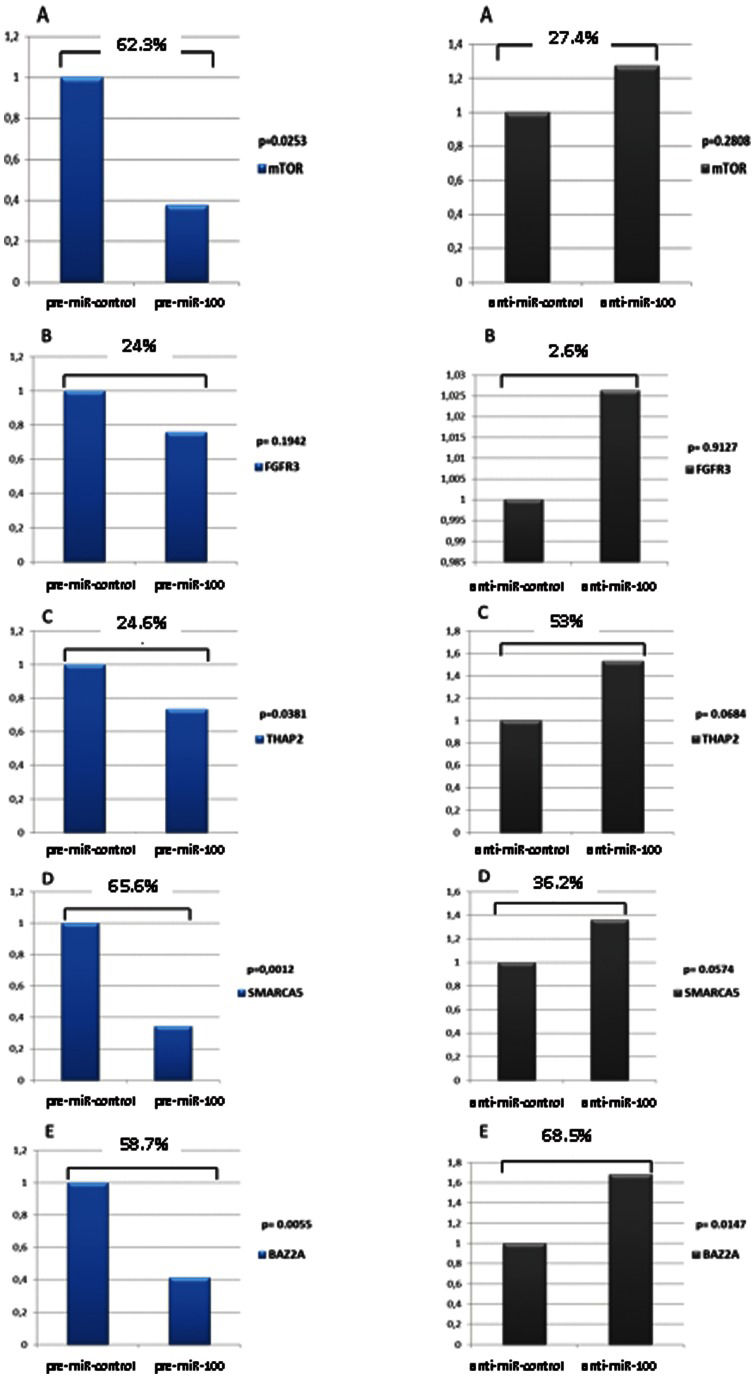
RESULTSThere was reduction in mTOR (p = 0.025), THAP2 (p = 0.038), SMARCA5 (p = 0.001) and BAZ2A (p = 0.006) mRNA expression in DU145 cells after exposure to miR-100. In PC3 cells, mTOR expression was decreased by miR-100 (p = 0.01). There was a reduction in the expression levels of proteins encoded by studied genes, ranging from 34% to 69%.
CONCLUSIONSWe demonstrate that miR-100 is a context-dependent miRNA controlling BAZ2, mTOR, FGFR3, SMARCA5 and THAP2 that might be involved in PC progression. The elucidation of the roles of miRNAs in tumors is important because they can be used as therapeutic targets in the future.
MicroRNAs (miRNAs) are small noncoding RNA molecules that are involved in many cellular processes, and some have recently been correlated with the development and progression of different tumors (1). We previously found that there is a reduction in expression of miRNA-100 during the transition from localized to metastatic prostate cancer (2). Yet, conversely, men with higher levels of miR-100 were more likely to suffer biochemical recurrence after radical prostatectomy (3). This discrepancy suggests that miR-100 is a context-dependent miRNA, acting sometimes as oncogene (oncomiR) and sometimes as tumor suppressor miRNA (tsmiR). Many genes that are predicted as targets of miR-100 have important roles in cell proliferation, survival and chromosome stability (http://atlasgeneticsoncology.org/Genes/MIR100ID51447ch11q24.html). The aim of this study was to determine the role of miR-100 in the control of these genes in prostate cancer cell lines.
METHODSThe prostate cancer cell lines DU145 and PC3 were maintained in McCoy's medium with 20% FBS and 1% antibiotic/antimycotic solution (Sigma Co, St. Louis, MO, USA) at 37°C in an atmosphere containing 5% CO2. Cells were grown in 24-well plates, with 5.0×104 cells seeded into each well. Transfections were carried out in Opti-MEM I with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), diluted 1:100 according to the manufacturer's protocol. Synthetic miR-100 (catalog number AM17100), antimiR-100 (catalog number AM17000) and their respective scrambled controls, control miR (ctrl miR; catalog number AM17110) and control antimiR (ctrl αmiR; catalog number AM17010) were purchased from Ambion (Austin, TX, USA). Negative controls and miRs were each used at 50 nM.
Total RNA for quantitative PCR was isolated with the Ambion mirVana kit (Austin, TX, USA) as recommended by the manufacturer. Quantitative PCR of mTOR, FGFR3, THAP2, SMARCA5 and BAZ2A was performed with TaqMan assays from Applied Biosystems (Foster City, CA, USA) using 2 μg RNA that was reverse transcribed using random primers and MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA, USA). After reverse transcription, all samples were subjected to DNaseI (Invitrogen) treatment to remove contaminating genomic DNA. All samples were amplified in a 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) and subjected to the comparative ‡‡Ct method by using human ß2 microglobulin as the internal standard. To calculate the difference in expression under different conditions, we used the following equations: ‡‡Ct = ‡Ct Pre-miR – ‡Ct Pre-miR negative control and ‡‡Ct = ‡Ct anti-miR – ‡Ct anti-miR negative control. All results were normalized to the control as 100%. The data shown are representative of three independent experiments. Statistical significance was calculated by two-tailed Student's t-test, and p<0.05 was considered significant. Statistical analyses were performed using GraphPad Prism version 5.
For Wester blotting, DU145 and PC3 cells (2.5×105 cells/well) were transfected with 50 nM of pre-miR 100 or its control. After 48 h, the cells were resuspended and lysed in their wells using RIPA buffer and protease inhibitor (Millipore, Billerica, MA, USA). Protein concentrations were established using the Pierce 660 nm Protein Assay (Thermo Scientific). Cell lysate samples containing 30 μg of protein were electrophoresed in a 12% SDS-polyacrylamide gel. After electrophoresis, the proteins were transferred to a PVDF Immobilon-P membrane (Millipore, Billerica, MA, USA). The membrane was blocked in Tris-buffered saline (TBS) containing 1% BSA (bovine serum albumin) (Sigma, St. Louis, MO, USA) and 1% Tween-20 for 15 sec at room temperature. The membranes were then incubated with primary antibody for 20 min in the SNAP i.d. protein detection system (Millipore) according to the manufacturer's instructions. The primary antibodies are described in Table 1. β-actin was used as a control. The secondary antibody was used at a dilution of 1:5000 with an incubation time of 20 min (Millipore) in the same instrument. The protein expression analysis was performed with Alliance 4.7 equipment (Uvitec, Cambridge, UK) using the Alliance 16.06 software.
RESULTSDU145 cells exposed to Pre-miR-100 showed a significant reduction in the mRNA expression of mTOR (62.3%, p = 0.025), THAP2 (24.6%, p = 0.038), SMARCA5 (65.6%, p = 0.0012) and BAZ2A (58.7%, p = 0.006). FGFR3 expression was reduced by 24%, but this change was not statistically significant (p = 0.194). Exposure to anti-miR-100 stimulated an increase in the expression of all of the genes tested, but the change was significant for only BAZ2A, which exhibited an increase of 68.5% (p = 0.0147) (Figure 1).
PC3 cells exhibited a significant reduction in the expression of mTOR after exposure to Pre-miR-100 (82.2%, p = 0.0103). The other four genes also showed reductions in expression, but this difference was not statistically significant. When exposed to anti-miR-100, all genes except SMARCA5 exhibited increased expression, but with no statistical significance (Figure 2).
Wester blot analysis showed that miR-100 negatively regulates mTOR, THAP2, SMARCA5 and BAZ2A and also causes a significant reduction in the protein expression of FGFR3, as shown in Figure 3. While miR-100 had no effect on the mRNA levels of these genes in PC3 cells, the expression of the corresponding proteins was significantly reduced after exposure to the miRNA (Figure 4).
Hundreds of microRNA target genes have been predicted by bioinformatics methods, but few have been substantiated by experimental assays. We have previously shown that miR-100 could be a context dependent miRNA that is differentially expressed during prostate cancer progression (2,3). In this study, we were able to show that miR-100 functions differently in two different prostate cancer cell lines and targets genes that can be classified as oncogenes or tumor suppressor genes.
The mTOR/PI3K/AKT pathway is related to many cellular processes, including cell proliferation and survival, and the deregulation of this pathway has been correlated with the progression of different tumors including prostate (4). The PI3Ks are enzymes involved in the phosphorylation of membrane inositol lipids to mediate cellular signal transduction (5). Receptor tyrosine kinases (RTKs) and some non-RTKs can activate PI3K, generating the second messenger PIP3 (phosphatidylinositol (3,4,5)-triphosphate). This activation drives a conformational change in AKT/PKB, resulting in the activation of both the mTORC1 and mTORC2 complexes and thus inducing cell survival, cell cycle progression and proliferation, cell migration, and angiogenesis. AKT is negatively regulated by the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10), which dephosphorylates PIP3. PTEN is often deleted in prostate cancer, and its deletion is associated with a high Gleason score (6) and castration-resistance status (7). We believe that the underexpression of miR-100 in metastatic prostate cancer and prostate cancer cell lines could act as a tsmiR, contributing to the upregulation of the mTOR pathway.
However, we have also shown that SMARCA5 and BAZ2A are also targets of miR-100. SMARCA5 is a member of the SWI/SNF protein family, which is involved in cell cycle regulation and signal transduction, facilitating the maintenance of heterochromatin by directing histone deacetylation during the cell cycle. The proper function of these SWI/SNF-like factors is required for accurate chromosome segregation during mitosis, preventing genomic instability and guaranteeing the efficient transmission of epigenetic information (8). Recently, SMARCA5 has been identified as a target of another member of the miR-100 family, miR-99a that is overexpressed in synovial sarcoma (9), confirming our findings.
BAZ2A, also known as TIP5 and WALp3, functions together with the SWI/SNF family member SNF2h to form the NoRC (nucleolar remodeling complex). This complex induces nucleosome sliding, which is important for transcription regulation (10). We believe that the overexpression of miR-100 in localized prostate cancer, which is related to biochemical recurrence, promotes the downregulation of both transcription control-related genes, leading to genetic instability, a well-known prognostic factor in prostate cancer (11,12). Although the biological roles of cellular THAP proteins remain unknown, some studies have shown that they are important in cell proliferation and cell cycle control. Bessière et al. recently showed that THAP1 is an endogenous physiological regulator of endothelial cell proliferation and G1/S cell cycle progression that modulates the expression of several pRb/E2F cell cycle target gene (13). We have confirmed the role of miR-100 as an oncomiR in localized prostate cancer, showing that it downregulates THAP2 (THAP domain containing apoptosis associated protein 2), thus increasing cell proliferation through the pRb pathway.
These results affirm that miR-100 is a context-dependent miRNA in prostate cancer that influences the fate of tumor cells; miR-100 sometimes acts as an oncomiR by impairing cell cycle control and promoting genomic instability by downregulating SMARCA5, BAZ2A and THAP2, while in other contexts it acts as a tsmiR, cooperating with other factors to downregulate the mTOR pathway.
The practical importance of our data is related to the possibility of using this miRNA as a targeted drug to treat neoplasias. Each miRNA should be studied in detail because miRNAs may have many target genes, and their context-dependence might produce broad effects that could be harmful to patients.
AUTHOR CONTRIBUTIONSLeite KR conceived and designed the study, was responsible for the analysis and interpretation of data, supervision, manuscript drafting, obtaining funds (FAPESP #2010-51207-6). Morais DR was responsible for the aquisition, analysis and interpretation of data. Reis ST was responsible for the statistical analysis. Viana N, Moura C, Florez MG and Dip N were responsible for the aquisition of data. Silva IA was responsible for the administrative, technical and material support. Srougi M was responsible for the critical revision of the manuscript for important intellectual content.
This study was supported by FAPESP (10/51207-6).
No potential conflict of interest was reported.