Liver transplantation has not increased with the number of patients requiring this treatment, increasing deaths among those on the waiting list. Models predicting post-transplantation survival, including the Model for Liver Transplantation Survival and the Donor Risk Index, have been created. Our aim was to compare the performance of the Model for End-Stage Liver Disease, the Model for Liver Transplantation Survival and the Donor Risk Index as prognostic models for survival after liver transplantation.
METHOD:We retrospectively analyzed the data from 1,270 patients who received a liver transplant from a deceased donor in the state of São Paulo, Brazil, between July 2006 and July 2009. All data obtained from the Health Department of the State of São Paulo at the 15 registered transplant centers were analyzed. Patients younger than 13 years of age or with acute liver failure were excluded.
RESULTS:The majority of the recipients had Child-Pugh class B or C cirrhosis (63.5%). Among the 1,006 patients included, 274 (27%) died. Univariate survival analysis using a Cox proportional hazards model showed hazard ratios of 1.02 and 1.43 for the Model for End-Stage Liver Disease and the Model for Liver Transplantation Survival, respectively (p<0.001). The areas under the ROC curve for the Donor Risk Index were always less than 0.5, whereas those for the Model for End-Stage Liver Disease and the Model for Liver Transplantation Survival were significantly greater than 0.5 (p<0.001). The cutoff values for the Model for End-Stage Liver Disease (≥29.5; sensitivity: 39.1%; specificity: 75.4%) and the Model for Liver Transplantation Survival (≥1.9; sensitivity 63.9%, specificity 54.5%), which were calculated using data available before liver transplantation, were good predictors of survival after liver transplantation (p<0.001).
CONCLUSIONS:The Model for Liver Transplantation Survival displayed similar death prediction performance to that of the Model for End-Stage Liver Disease. A simpler model involving fewer variables, such as the Model for End-Stage Liver Disease, is preferred over a complex model involving more variables, such as the Model for Liver Transplantation Survival. The Donor Risk Index had no significance in post-transplantation survival in our patients.
Liver transplantation is the treatment of choice for many terminal hepatic diseases, for which this treatment increases survival rates and improves quality of life. The 1- and 5-year survival rates following liver transplantation are currently 80% and 50%, respectively 1,2. Progressive improvement in transplantation results has led to an increase in the number of patients for which organ transplantation is indicated. However, the insufficient availability of donated organs has limited the number of procedures that can be performed 1–5. Because of the growing discrepancy between the numbers of organ donors and of potential recipients, it has become necessary to establish operative criteria for selecting the patients and the donors. Accordingly, a standardized system for classifying the severity of the patient's condition as well as the patient's prognosis has become necessary for optimizing outcomes.
Various medical scoring systems have been used as prognostic models of disease severity. For liver disease, the most widely used model is the Child-Turcotte classification modified by Pugh et al. 6. The Model for End-Stage Liver Disease (MELD) scoring system 7 was adopted in the USA for organ allocation in 2002 and in Brazil in 2006 8. Although the MELD is a well-established scale for predicting the mortality of patients on transplant waiting lists, some questions remain, particularly with regard to the risk of death and the relationship between the MELD score and post-transplantation survival 5,9,10. The Donor Risk Index (DRI) model 11, which was created via an analysis of 90,882 donors (UNOS/OPT N-USA), identified seven independent significant risk factors for graft failure in adult recipients. Finally, the Model for Liver Transplantation Survival (MLTS), which predicts post-transplantation survival, was developed as a strong predictor that applies pre-transplantation variables regarding the donor, the recipient and the surgical team 12; however, studies and discussion in the medical literature regarding the MLTS are limited.
Organ allocation should be designed not only to avoid death before transplantation, but also to prevent premature post-transplantation deaths and, thus, the waste of scarce resources. The development of a model capable of predicting post-transplantation survival has become a matter of vital interest to the transplant community because a system for allocating organs that balances disease severity with anticipated results can maximize the survival benefits to transplant patients. A model that can reliably predict patient survival after a liver transplant is essential. However, it is possible that other variables that are not considered might affect survival and reduce the capacity of a particular model to generate reliable predictions. For this reason, each model must be validated across multiple centers to confirm its efficacy. Our aim was to compare the MELD, MLTS, and DRI prognostic scores relative to post-operative survival outcomes in adult patients receiving a liver transplant from a cadaver donor.
METHODSWe performed a retrospective study analyzing the data from 1,270 patients who received a liver transplant performed at 15 institutions in the state of São Paulo, Brazil, between July 2006 and July 2009 following approval by the Santa Casa Ethics Committee. Of these 1,270 cases, the following were excluded from the study:
- •
Transplants performed on patients younger than 13 years of age (144/1,270; 11.3%), who were evaluated using the Pediatric End–Stage Liver Disease scoring system.
- •
Transplants in patients with acute liver failure, wherein the criteria of urgency were based on the Kings College or Clichy models; 92/1,270 (7.2%) patients were evaluated using these models.
- •
Transplants on patients with amyloid polyneuropathy who received “domino” transplants from live donors (21/1,270; 1.6%).
- •
Transplants from donors whose data were incomplete; 7/1,270 (0.6%) of the cases had incomplete data for the donors in the database of the Health Department of the State of São Paulo.
After excluding the aforementioned cases, our final case sample consisted of 1,006 transplant patients with an average age of 51 (13–74) years. Only 30% of the patients were female. The three most frequent liver disease diagnoses of the transplant patients were as follows: Child B or C liver cirrhosis (63.5%), hepatocellular carcinoma (16.3%) and Child A liver cirrhosis (4.5%).
From the records of the remaining 1,006 patients, we collected the data of interest to the study and the variables that constituted the MELD, MLTS, and DRI scores prior to transplantation. In particular, the following data were obtained from the Health Department of the State of São Paulo – which is responsible for collecting and analyzing data from these 15 transplant centers – and were recorded in a Microsoft Excel 2007® spreadsheet:
- •
Recipient data: the record number, age, sex, blood type, diagnosis, calculated and corrected MELD scores, total bilirubin level, international normalized ratio (INR) for blood clotting, sodium level, dialysis, and transplant data;
- •
Donor data: the record of whether the liver was split, race, place, age, cause of death, sex, height, and weight; and
- •
Surgery data: the period of cold ischemia (in hours) and the period of warm ischemia (in min).
We calculated the MELD, MLTS, and DRI scores for the final 1,006 patients included in the study using Microsoft Excel 2007®. Other data of interest to the study included the retransplant status and the survival duration, which was measured according to the interval between the transplant and the date of the last record or the date of death as recorded by the transplant headquarters of the Health Department of the State of São Paulo.
Statistical analysisA Cox proportional hazards (CoxPH) model was used to identify the relevance of the MELD, MLTS, and DRI scores to patient mortality. The results were expressed as CoxPH values with a 95% confidence interval (CI95%). Kaplan-Meier survival curves were used to analyze the survival trends over time. The power of the MELD, the DRI, and the MLTS to predict mortality was evaluated by calculating the area under the receiver operating characteristic (ROC) curve, in which a value of 1 represents perfect discrimination whereas a value of 0.5 represents discrimination that is no more accurate than chance. Unless otherwise noted, the mean values are reported with standard deviations (SDs). Statistical significance was accepted at p<0.05. All analyses were performed using SPSS for Windows® version 13.0, with the exception of the comparison of the ROC curves, which was analyzed using Analyse-it® 2.12 software.
RESULTSStudy characteristicsThe means (SDs) of the components of the MELD and MLTS score formulas were as follows: creatinine level: 1.56 (±1.40) mg/dl; total bilirubin level: 8.29 (±11.31) mg/dl; INR: 2.10 (±1.48); prothrombin time: 24.81 (±14.80) seconds; donor age: 40.79 (±16.10) years; warm ischemia duration: 49.59 (±15.71) min; and cold ischemia duration: 8.57 (±2.73) hours. The median MELD score was 22.17 (±11.24), and the median MLTS score was 2.07 (±0.74).
The donors had a mean DRI score of 1.44±0.34 and a mean height of 168.00±12.24 cm. The following parameters were the most frequent: an age range of 0–39 years (41.8%) and white race (68.29%); the incidence of a split liver was only 4.6%. The principal cause of death was stroke (57.75%), and in 86.8% of the cases, the donor and the transplant recipient were from the same area.
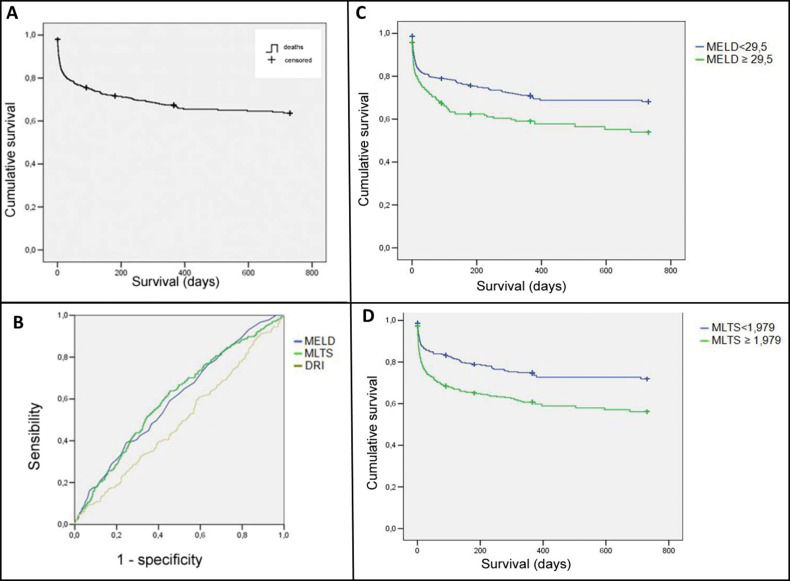
Analysis of the survival rate and the prognostic factorsOf the 1,006 patients included in this study, 274 (27%) died, and the average mortality rate was 0.03 patients per month based on analysis of the Kaplan-Meier survival curve (Figure 1A).
A) The Kaplan-Meier curve for the survival of 1,006 patients who received a liver transplant in the state of São Paulo between 2006 and 2009. B) The ROC curves of the MELD, MLTS, and DRI scores for 2-year mortality for 1,006 liver transplants performed in the state of São Paulo between 2006 and 2009. C) The Kaplan-Meier curves for mortality according to the MELD scores for 1,006 liver transplants performed in the state of São Paulo between 2006 and 2009. D) The Kaplan-Meier curves for mortality according to the MLTS scores for 1,006 liver transplants performed in the state of São Paulo between 2006 and 2009.
We analyzed the relationships between mortality and the MELD, MLTS, and DRI scores. We performed univariate survival analyses using a CoxPH model and hazard ratios (HRs) to identify whether the MELD, MLTS, and DRI scores were significant prognostic factors for the death of the patients included in this sample (Table 1). The areas below the ROC curves for the MELD, MLTS, and DRI scores were compared for the 7-day, 1-month, 3-month, 6-month, 1-year, and 2-year post-transplantation survival ratios. The results of these analyses are presented in Table 2. The ROC curves for 2-year mortality are shown in Figure 1B.
The results of univariate survival analysis of the Model for End-Stage Liver Disease, Model for Liver Transplantation Survival, and Donor Risk Index scores for 1,006 liver transplants performed in the state of São Paulo between 2006 and 2009.
| Model | B | Standard error | Wald X2 | CoxPH | CI95% (CoxPH) | p-value |
|---|---|---|---|---|---|---|
| MELD | 0.02 | 0.01 | 21.28 | 1.02 | 1.01 to 1.03 | <0.001* |
| MLTS | 0.36 | 0.08 | 19.33 | 1.43 | 1.22 to 1.68 | <0.001* |
| DRI | 0.07 | 0.18 | 0.17 | 1.08 | 0.76 to 1.53 | 0.677 |
*statistically significant
Comparison of the survival rate relative to the Model for End-Stage Liver Disease, Model for Liver Transplantation Survival and Donor Risk Index scores based on the areas under the Receiver Operating Characteristic curve for 1,006 liver transplants performed in the state of São Paulo between 2006 and 2009.
| Time point and model | N° deaths | Area | CI95% (area) | p-value | ||
|---|---|---|---|---|---|---|
| 7 days | 117 | |||||
| MELD | 0.59 | 0.54 to 0.65 | 0.001* | |||
| MLTS | 0.59 | 0.54 to 0.64 | 0.002* | |||
| DRI | 0.46 | 0.40 to 0.52 | 0.150 | |||
| 1 month | 177 | |||||
| MELD | 0.58 | 0.53 to 0.62 | 0.001* | |||
| MLTS | 0.59 | 0.55 to 0.64 | <0.001* | |||
| DRI | 0.46 | 0.42 to 0.51 | 0.122 | |||
| 3 months | 215 | |||||
| MELD | 0.61 | 0.57 to 0.65 | <0.001* | |||
| MLTS | 0.62 | 0.58 to 0.66 | <0.001* | |||
| DRI | 0.47 | 0.42 to 0.51 | 0.155 | |||
| 6 months | 243 | |||||
| MELD | 0.61 | 0.57 to 0.65 | <0.001* | |||
| MLTS | 0.60 | 0.56 to 0.65 | <0.001* | |||
| DRI | 0.47 | 0.42 to 0.51 | 0.107 | |||
| 1 year | 264 | |||||
| MELD | 0.60 | 0.56 to 0.64 | <0.001* | |||
| MLTS | 0.60 | 0.56 to 0.64 | <0.001* | |||
| DRI | 0.48 | 0.44 to 0.52 | 0.312 | |||
| 2 years | 274 | |||||
| MELD | 0.60 | 0.56 to 0.64 | <0.001* | |||
| MLTS | 0.60 | 0.56 to 0.64 | <0.001* | |||
| DRI | 0.49 | 0.45 to 0.53 | 0.724 | |||
*statistically significant
Based on the ROC curves for the MELD, MLTS, and DRI scores for 2-year survival, we calculated the following cutoff values:
- •
MELD: ≥29.5 (sensitivity of 39.1% and specificity of 75.4%);
- •
MLTS: ≥1.979 (sensitivity of 63.9% and specificity of 54.5%); and
- •
DRI: ≥2.253 (sensitivity of 4.4% and specificity of 98.6%).
The Kaplan-Meier survival curves significantly differed between the patients with scores lower than or equal to the cutoff values for the MELD and the MLTS and those with scores greater than the respective cutoff values. For the MELD and the MLTS, the mortality rate of the patients with scores equal to or above the cutoff value was poorer than that of those with scores below the cutoff value (Figures 1C and 1D).
Multivariate analysis revealed that the most important determining factor for mortality according to the MELD score was the total bilirubin level. For the MLTS score, the most important determining factors were the total bilirubin level, the cold and warm ischemia durations, and the retransplant status (Table 3).
Multivariate survival analysis of the parameters used to calculate the Model for End-Stage Liver Disease and Model for Liver Transplantation Survival scores as independent variables for 1,006 liver transplants performed in the state of São Paulo between 2006 and 2009.
| Variable | β | SE† | Wald X2 | CoxPH | CI95% (CoxPH) | p-value |
|---|---|---|---|---|---|---|
| MELD | ||||||
| Log-creatinine (mg/dL) | 0.17 | 0.09 | 3.69 | 1.19 | 1.00 to 1.42 | 0.055 |
| Log-total bilirubin (mg/dL) | 0.12 | 0.06 | 4.18 | 1.13 | 1.01 to 1.27 | 0.041* |
| INR | 0.18 | 0.16 | 1.27 | 1.19 | 0.88 to 1.62 | 0.260 |
| MLTS | ||||||
| Recipient age | 0.01 | 0.00 | 1.92 | 1.01 | 1.00 to 1.02 | 0.166 |
| Donor age | 0.00 | 0.00 | 0.02 | 1.00 | 0.99 to 1.01 | 0.897 |
| Log-creatinine (mg/dL) | 0.38 | 0.22 | 3.06 | 1.46 | 0.96 to 2.22 | 0.080 |
| Log-total bilirubin (mg/dL) | 0.26 | 0.13 | 3.92 | 1.29 | 1.00 to 1.67 | 0.048* |
| Cold ischemia duration (hours) | 0.05 | 0.02 | 4.74 | 1.05 | 1.00 to 1.09 | 0.029* |
| Warm ischemia duration (min) | 0.01 | 0.00 | 11.88 | 1.01 | 1.00 to 1.02 | 0.001* |
| Log-prothrombin time (s) | 0.01 | 0.00 | 2.89 | 1.01 | 1.00 to 1.01 | 0.089 |
| Retransplant | 0.53 | 0.20 | 7.15 | 1.70 | 1.15 to 2.50 | 0.008* |
The most significant of our findings was that the MLTS yielded a similar outcome to that of the MELD for predicting post-transplantation mortality (both p=0.001). However, the DRI did not show a statistically significant association with post-transplantation mortality (p=0.677).
MELDThe MELD is a well-established (area under the ROC curve=0.78–0.87) method for estimating the 3-month survival of patients who do not receive a transplant 5–13. However, its association with post-transplantation survival is not clear, and its degree of superiority for non-transplanted patients is modest and limited 14,15. If the MELD could simultaneously predict and identify those patients who are at an elevated risk of dying post-transplantation, its use for allocation would be supported. The ideal system should not only define the probability of death without transplantation, but also predict the risk of death post-transplantation, thus increasing its utility 16.
The MELD has been reported to predict 3-month mortality in most candidates on transplant waiting lists (83–87%), reducing the mortality rate by approximately 3.5% and increasing the number of transplants for diseases such as hepatocellular carcinoma by approximately 10%, ranging from 3.1 to 22% 17,18. Despite its many advantages, the MELD does not precisely predict survival in 15–20% of cases. The addition of variables that are good determinants of liver and kidney function may improve the precision of this model 17.
In this study, we compared the areas under the ROC curve for post-transplantation survival for the MELD score at 7 days, 1 month, 3 months, 6 months, 1 year and 2 years after transplantation. The areas under the curve for all of these time intervals indicated that the MELD-based predictions were more accurate than chance (i.e., >0.5). This model is consistently described in the literature as showing good accuracy (c-statistic=0.78–0.87) in estimating the survival of patients not receiving a liver transplant. However, the MELD may be a poor model for predicting post-transplantation survival, as shown in the study by Jacob in 2004 (c-statistic=0.58) 13. In a study examining 2,565 transplants from cadaver donors, Desai et al. similarly found that the MELD showed weak accuracy for predicting 3-month survival (c-statistic of 0.54; CI95%=0.50–0.59) and 1-year survival (c-statistic of 0.55; CI95%=0.52–0.59) following transplantation 19. In this study, the MELD served as a significant predictor of post-transplantation death (ROC curve analysis c-statistic=0.59?0.60; CoxPH model: p<0.001).
However, our ROC curve for the MELD did not yield a highly accurate level of agreement (c-statistic=0.8–0.9). These findings are consistent with those in the literature, as the MELD is well established for predicting the survival of those on transplant waiting lists but remains controversial for post-transplantation survival prediction. For example, our findings were similar to those from a study of 121 patients by Nagler et al., who obtained a c-statistic for the MELD of 0.61 and showed a relationship between the MELD score and post-transplantation survival 20.
DRIDonor characteristics are important factors in determining transplant outcomes. The DRI considers seven donor characteristics using the Cox regression model; a lower DRI score indicates an organ that is closer to ideal for transplantation 21. Feng et al. 11 summarized the ideal characteristics of a cadaver donor. Briefly, they found that the ideal donor was young (<40 years old), was healthy, was tall (≥1.70 m), experienced brain trauma, was not used for split liver transplantation and was not a donor after cardiac death (DCD). In addition, the ideal donor had stayed <4 days in intensive care, had an average blood pressure ≥60 mmHg, had no requirement for vasoactive drugs, and had laboratory findings within the following limits: bilirubin ≤2 mg/dl, alanine level <170 U/L, aspartate aminotransferase level <140 U/L, and blood sodium level <160 mEq/L 2,9,21.
In our study, the DRI was not a good indicator of post-transplantation mortality (CoxPH=1.08, p=0.677) or survival (the areas under the ROC curve for 3-month and 1-year survival had c-statistic values of 0.47 and 0.48, respectively; these values were always <0.5). These negative findings might be due to the heterogeneity of the Brazilian population. Additionally, there may have been influential factors that are not included in the DRI.
Few studies have analyzed the DRI as a post-transplantation survival index. However, Northup et al. investigated the effect of an expanded set of donor criteria on retransplantation outcomes and developed an index based on the DRI. Among the 1327 retransplant recipients examined, 611 (46%) received a graft involving at least one expanded criterion. They found that the DRI was a good indicator of post-retransplantation survival (HR 2.19, CI95%=1.63–2.94; p<0.0001). Moreover, when the cause of graft failure was included in the DRI, the prediction strength significantly increased (HR 2.49, CI95%=1.89–3.27; p<0.0001) 22.
The interactions between the DRI and the MELD have been examined by some authors. In a study involving 1,090 transplants, Bonney et al. 23 found that patients with low to intermediate MELD scores (i.e., patients with low to moderate disease severity) only experienced a survival benefit from transplantation when they received an organ corresponding to a low DRI score, whereas recipients with a high MELD score (severely ill) benefited from transplantation regardless of whether the donor organ corresponded to a low or high DRI score. These results partially corroborated those of Schaubel et al. 24, who demonstrated that patients with a high DRI score that received a transplant had a mortality rate 3.5-fold greater than those that remained on the waiting list. These results further established that severely ill patients with a high MELD score benefited from receiving an organ, even from a donor with a high DRI score, whereas less gravely ill patients with a low MELD score did not benefit from receiving an organ from a donor with a high DRI score compared to remaining on the waiting list.
To date, no extensive information is available regarding the relationship between the characteristics of liver donors and the post-transplantation survival of organ recipients. However, this issue should be considered further, as it could become an important consideration for transplant teams and transplant candidates as decisions are made to optimize organ allocation and organ acceptance.
MLTSTransplant outcomes depend on the interaction of the following three factors: the donor, the recipient, and the perioperative period. The MLTS is a mathematical model created by Ghobrial et al. 12 that is based on these factors. The MLTS uses multivariate statistical analysis to independently determine the impact of these factors on transplant recipient survival. In this study, we constructed ROC curves for the MLTS data and found that for all time intervals examined, the areas under the ROC curve were higher than 0.5. The areas under the ROC curve for our MELD and MLTS datasets were not significantly different (Table 2). Prior studies by Jacob et al. 13,14 produced MLTS c-statistic values for 3-month, 6-month, and 1-year post-transplantation survival of 0.57, 0.57, and 0.56, respectively, indicating a weak discriminatory power for predicting survival in their study population.
Scores to predict survival after liver transplantation are indeed an interesting and current topic, and one limitation of our study was not including and comparing the most recently developed scores, such as the Survival Outcome Following Liver Transplantation score, the balance of risk score, and the multi-layer perception network, with the examined models 25–27. These scores are not widely used to predict survival after liver transplantation; however, we believe that further studies should be conducted to create scores in each country or region or even to adapt the already existing scores to produce scores that are more appropriate for the characteristics of each local setting.
In conclusion, the present results for 1,006 liver transplants performed between 2006 and 2009 using cadaver donors in the state of São Paulo, Brazil, showed that the MELD and the MLTS display a similar ability to predict death after a liver transplant and that the DRI does not predict mortality after a liver transplant. In principle, a model that uses recipient, donor, and surgical team variables, such as the MLTS, would be expected to be preferable to a model based solely on the donor variables (i.e., the DRI) or the recipient variables (i.e., the MELD). However, the present results showed that the MLTS did not provide more reliable information than the MELD. Therefore, we suggest that it would be preferential to continue to employ a simpler model involving fewer variables, such as MELD, rather than adopt a more complex model involving more variables, such as the MLTS, while creating a new local or regional score.
AUTHOR CONTRIBUTIONSAranzana EM performed the research and collected and analyzed the data. Ferreira FG contributed to the design of the study and wrote the manuscript. Ribeiro MA, Coppini AZ, Massarollo PCB and Szutan LA contributed to the design of the study and the analysis of the data.
No potential conflict of interest was reported.