Aerobic exercise training prevents cardiovascular risks. Regular exercise promotes functional and structural adaptations that are associated with several cardiovascular benefits. The aim of this study is to investigate the effects of swimming training on coronary blood flow, adenosine production and cardiac capillaries in normotensive rats.
METHODS:Wistar rats were randomly divided into two groups: control (C) and trained (T). An exercise protocol was performed for 10 weeks and 60 min/day with a tail overload of 5% bodyweight. Coronary blood flow was quantified with a color microsphere technique, and cardiac capillaries were quantified using light microscopy. Adenine nucleotide hydrolysis was evaluated by enzymatic activity, and protein expression was evaluated by western blot. The results are presented as the means ± SEMs (p<0.05).
RESULTS:Exercise training increased the coronary blood flow and the myocardial capillary-to-fiber ratio. Moreover, the circulating and cardiac extracellular adenine nucleotide hydrolysis was higher in the trained rats than in the sedentary rats due to the increased activity and protein expression of enzymes, such as E-NTPDase and 5′-nucleotidase.
CONCLUSIONS:Swimming training increases coronary blood flow, number of cardiac capillaries, and adenine nucleotide hydrolysis. Increased adenosine production may be an important contributor to the enhanced coronary blood flow and angiogenesis that were observed in the exercise-trained rats; collectively, these results suggest improved myocardial perfusion.
Exercise training is an important factor for preventing cardiovascular risk and has been associated with several cardiovascular benefits. To characterize the benefits of exercise, resting bradycardia has been considered to be the hallmark of cardiovascular effects for exercise-training adaptation in animals.1,2 Left ventricular hypertrophy and angiogenesis also result from aerobic exercise training and are considered physiologically beneficial to the heart by improving myocardial perfusion and function.3–5
Adenosine is one of the principal factors that regulates tissue function, particularly when the energy supply fails to meet the cellular energy demand. In this manner, adenosine accumulates during ischemia or hypoxia due to the imbalance between oxygen supply and demand.6 During exercise, myocardial oxygen demand increases, and adenosine release is increased to levels that are similar to those of restricted oxygen supply conditions.7,8 One important factor that contributes to adenosine accumulation is an increase in the substrate from which the nucleoside is formed. Therefore, the cleavage of ATP and the subsequent increase in AMP concentration, associated with an increase in the activity of the adenosine-forming enzymes (nucleotidases), lead to the accumulation of adenosine to increase the energy supply by vasodilatation and to decrease the energy demand through a negative-feedback mechanism.6
Adenine nucleotides are continuously present in the extracellular space of the heart. Extracellular ATP is considered to be a powerful signaling molecule, and a variety of extracellular enzymes utilize this nucleotide to induce biological responses. The release of endogenous nucleotides represents a critical component for the initiation of a signaling cascade. An important route of nucleotide appearance in the extracellular milieu is nucleotide efflux via various pathways, which include ATP release channels, nucleotide-specific transporters and vesicular exocytosis.9 The mechanism of sequential ATP hydrolysis via the conversion of ADP into AMP is mediated by a family of Ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDases 1-8) enzymes, which have been classified in order of their discovery and classification. E-NTPDases 1, 2, 3, and 8 are expressed as cell surface enzymes, E-NTPDases 5 and 6 exhibit intracellular localization and undergo secretion after heterologous expression, and E-NTPDases 4 and 7 are exclusively intracellular. Significant homology has been confirmed between E-NTPDase 1 and human CD39. Moreover, 5′-nucleotidase, which is otherwise known as CD73, hydrolyses 5′-AMP and is expressed in different tissues, including the heart, where it is located both in the extracellular and intracellular compartments.9 Changes in the activities of ectonucleotidases may modify the production of adenosine and affect myocardial blood flow. In fact, increased 5′-nucleotidase enzyme activity has been observed following a single session of endurance and sprint training,10 and increased ecto-5′-nucleotidase activity in the rat heart was also observed following a higher intensity program of chronic swimming training.11 The increase in enzyme activity that was observed in these studies suggests alterations in the production of adenosine, but the relationships of these alterations with blood flow have not yet been investigated.
Adenosine, which is produced primarily through the metabolism of ATP, interacts with adenosine receptors (the four subtypes of ARs are A1, A2A, A2B, and A3), which are G-protein-coupled receptors. The divergence of the coupling between each receptor and various G proteins, which are linked to different enzymes, channels or transporters, can elicit varied responses in different tissues or cells. Multiple cardiac cells, including fibroblasts, endothelial cells, smooth muscle cells, and myocytes, express adenosine receptors. Among other effects, these G-protein-coupled receptors in the heart mediate responses of coronary flow modulation and cardioprotection.12
Therefore, the present investigation was intended to verify whether a chronic, low-intensity swimming training protocol could alter coronary blood flow, cardiac capillaries and the hydrolysis of extracellular adenine nucleotides in normotensive rats.
METHODSAnimalsMale Wistar rats (weighing 180-250 g) were used in these experiments. The rats were randomly divided into control (C) and trained (T) groups and were kept separated in standard cages. Food and water were provided ad libitum. The room temperature was maintained at 23±1°C, and a 12:12 hours light-dark cycle was maintained throughout the experiment. The rats were identified and weighed weekly to determine their adaptation to the workload. The animal protocol was conducted according to the Guideline for the Care and Use of Laboratory Animals and was approved by the Ethics Committee of the School of Physical Education of the University of São Paulo (No. 2006/05).
Exercise Training ProtocolThe swimming training was performed as previously described;1 it was executed five times each week with a duration of 60 min in a swimming system with warm water at 30-32°C for 10 weeks. Adaptation sessions were performed for the first two weeks of the protocol; during these sessions, the exercise duration and workload were gradually increased until the rats could swim for 60 min while wearing caudal dumbbells that were 5% of their body weight. The adaptation sessions avoids the excessive physiological stress observed on the immune system.13 This protocol is defined as low- to moderate-intensity and long-term training, which is effective for the promotion of cardiovascular adaptations and increases in muscle oxidative capacity.14
Hemodynamic and Blood Flow AnalysisCoronary blood flow was evaluated by the colored microsphere technique, which was previously described.15 Briefly, 24 hours after the last exercise session, the rats were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg, ip) to facilitate the implantation of two catheters that were filled with saline solution into the femoral artery and left ventricle. A PE-10 catheter was positioned into the abdominal aorta through the femoral artery for the direct measurement of arterial pressure (AP). The second PE-50 catheter was inserted into the left ventricle through the right carotid artery for colored microsphere infusion. The catheter position was determined by observing the characteristic left ventricular pressure waveform during surgery. To show that the aortic valves were not injured, arterial pressure was measured before and after ventricular catheterization. If the diastolic arterial pressure decreased, suggesting an aortic valve lesion, the animal was discarded. The catheters were anchored with silk sutures and exteriorized at the back of the neck. Rats that received food and water ad libitum were studied one day after the procedure, and the animals were conscious and allowed to move freely during the experiments.16
The femoral artery catheter was connected to a pressure transducer, and arterial pressure signals were monitored continuously, except during microsphere infusion and withdrawal of a reference blood sample. The recorded data were analyzed on a beat-to-beat basis to quantify changes in the heart rate and the systolic, diastolic and mean blood pressure. Dye-Trak colored microspheres (15 μm; Triton Technology, San Diego, CA, USA) were infused at rest (red: 200 000 CM) for blood flow and cardiac output determination. Microsphere infusion, reference blood samples and tissues were processed according to the method of Hakkinen et al.15 After the microsphere infusion, the animals were sacrificed, and their hearts were removed to determine blood flow. The absorption spectrum peak for the red microspheres was obtained at 530 nm, and the minimum acceptable measurement was 0.010 absorbance units.
For each infusion, the tissue flow rates were calculated according to the following formula:
where Qt and Qb represent the flow in the sample tissue and in the reference blood, respectively, and At and Ab represent the peak absorbance of the tissue sample and of the reference blood, respectively. The calculation of Qb, in mL.min-1, was:
where 1.05 g.mL-1 is the specific gravity of blood, and 0.5 mL.min-1 is the withdrawal rate. The blood flow rates were divided by the tissue weights to yield mL.min-1.g-1.
Adenine Nucleotides and Nucleoside HydrolysisIsolation of Blood Serum FractionThe blood samples were drawn following decapitation, and they were subsequently centrifuged in plastic tubes at 5,000 x g for 15 min at 4°C. The serum samples were stored at -20°C until they were used in the experiments.
Isolation of Sarcolemmal FractionThe sarcolemmal preparation was isolated from the rat hearts as described by Velema and Zaasgma.17 The ventricles were minced and homogenized in 80 mL of 20 mM Tris-HCl that contained 1.0 mM EDTA, pH 7.0, using a tissue homogenizer for four periods of 7-s periods at maximum speed with 15-s resting intervals. The homogenate was centrifuged for 20 min at 8,800 x g. The supernatant (S1) was centrifuged for 20 min at 12,500 x g. This step was repeated with supernatant S2, and the resulting supernatant S3 was recentrifuged for 60 min at 44,000 x g. The obtained pellet (P4) was resuspended in 15 mL of 20 mM Tris-oxalate, 0.6 M KCl and 1.0 mM EDTA, pH 6.8, and was centrifuged for 60 min at 44,000 x g. The pellet obtained from this final centrifugation step (sarcolemmal fraction) was suspended in 20 mM Tris-HCl and stored at -20°C. This preparation was used as the enzyme source. All of the procedures were performed at 4°C.
Measurement of ATP and ADP HydrolysisATP and ADP hydrolysis were determined using a modification of the method described by Yegutkin.18 The enzyme activity was routinely determined at 37°C in the following incubation media: (a) the blood serum was incubated with 112.5 mM Tris-HCl, pH 8.0, with approximately 1.0 mg of serum protein at 37°C for 40 min in a final volume of 0.2 mL, and the reaction mixture contained ADP or ATP as a substrate19 (2.0 mM or 3.0 mM, respectively); (b) the cardiac sarcolemmal fraction was incubated with 50 mM Tris-HCl buffer, pH 7.5, 1.5 mM CaCl, and 0.8–1.0 μg of protein in a final volume of 0.2 mL, and the reaction mixture contained ADP or ATP as a substrate20 (3.0 mM or 2.0 mM, respectively).
Both of the reactions were initiated by adding the substrate to the reaction mixture, which was preincubated for 10 min at 37°C. The incubation times and protein concentrations were chosen to ensure the linearity of the reaction. The reactions were stopped by the addition of 0.2 mL of 10% trichloroacetic acid (TCA). The serum samples alone were centrifuged at 5,000 x g for 15 min to eliminate any precipitated protein, and the supernatant was used for the colorimetric assay. All of the samples were chilled on ice, and the amount of inorganic phosphate (Pi) that was liberated was measured according to the procedure of Lanzetta et al.21 To correct for non-enzymatic hydrolysis, we performed controls by adding the serum after the reaction was stopped with TCA. The enzyme activities were expressed as nanomoles of Pi released per minute per milligram of protein.
Measurement of AMP HydrolysisThe enzyme activity was routinely determined at 37°C in the following incubation media: (a) in the blood serum, the reaction mixture, which contained AMP as a substrate (2.0 mM) in 100 mM Tris–HCl, pH 7.5, was incubated with 1.0-1.5 mg serum protein at 37°C in a final volume of 0.2 mL;22 (b) in the cardiac sarcolemmal fraction, the reaction mixture, which contained AMP (2.0 mM) as a substrate in 100 mM Tris-HCl buffer, pH 7.5, and 1.5 mM MgCl was incubated with 1-2 μg of protein in a final volume of 0.2 mL. All of the other procedures were the same as for the ATP-diphosphohydrolase activity, which was described above.
The protein concentration was measured according to the method of Bradford,23 and bovine serum albumin was used as a standard.
Western Blot AnalysisThe protein levels of E-NTPDase 1 (CD 39) and ecto-5′-nucleotidase (CD 73) in the heart muscle were analyzed by western blotting. The frozen heart muscles (100 mg) were homogenized in cell lysis buffer that contained 100 mM Tris-HCl, 50 mM NaCl, 1% Triton X-100, and a protease inhibitor cocktail (1:100, Sigma Aldrich, Saint Louis, MO, USA). Insoluble heart tissues were removed by centrifugation at 3,000 x g at 4°C for 10 min. The samples were subjected to SDS-PAGE (10%). After electrophoresis, the proteins were electro-transferred onto a nitrocellulose membrane (BioRad Biosciences, NJ, USA). Equal loading of the samples (50 μg) and an even transfer efficiency were monitored with the use of 0.5% Ponceau S staining of the blot membrane. The blot membrane was then incubated in a blocking buffer (5% non-fat, dry milk; 10 mM Tris-HCl, pH 7.6; 150 mM NaCl; and 0.1% Tween-20) for 2 h at room temperature. The membrane was then incubated overnight at 4°C with a rabbit polyclonal antibody directed against CD39 and CD73 (1:1,000; Santa Cruz Biotechnology, CA, USA). The binding of the primary antibody was detected with the use of peroxidase-conjugated secondary antibodies, and enhanced chemiluminescence reagents (Amersham Biosciences, NJ, USA) were used to visualize the autoradiogram, which was later exposed to photographic film. The film was developed, and the bands were analyzed using the Scion Image software program (Scion Corporation, based on the NIH image program). The heart muscle GAPDH expression levels were used to normalize the results. The results are expressed in arbitrary units (AU).
Cardiac Morphometric AnalysisTwenty-four hours after the last exercise session, the rats were sacrificed by decapitation, and their hearts were immediately excised and rinsed in physiological solution, dried on filter paper and weighed. For the morphometric analysis, the LV was fixed in 6% formaldehyde and embedded in paraffin; it was cut into 5-μm sections at the level of the papillary muscle and subsequently stained with Periodic Acid Schiff (PAS) to visualize the cellular structures. From each sample, three microscopic fields were randomly chosen from the transversal sections using a light microscope (with an oil immersion objective, x400 magnification). The capillaries were identified as small vessels, and they contained a uniform lumen with a diameter <12 μm, which was previously described.5 The quantification of the capillaries was determined in microscopic fields that measured 0.04 mm2, and the measurement was expressed as a capillary-to-fiber ratio (number of capillaries per myocyte).
Statistical AnalysisThe data are reported as the mean ± standard error of the mean (SEM). An unpaired Student's t-test was used to compare the means. To indicate how closely two variables changed in relationship to each other, Pearson's correlation coefficient was used. For all of the analyses, values of p<0.05 were considered to be significant.
RESULTSArterial Pressure, Heart Rate and Coronary Blood FlowAlthough the systolic, diastolic, and mean blood pressures were not different between the untrained and trained groups, the exercise training significantly decreased the resting heart rate, as demonstrated in Table 1.
Hemodynamic parameters.
| SBP | DBP | MBP | HR | |
|---|---|---|---|---|
| mm Hg | mm Hg | mm Hg | bpm | |
| C (n = 5) | 115.9±3.5 | 94.5±1.6 | 107.2±2.1 | 328.5±7.3 |
| T (n = 5) | 118.3±6.4 | 87.7±4.7 | 103.4±5.1 | 285.8±6.7∗ |
Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP) and heart rate (HR) are presented for the control (C) and trained (T) groups. The values are represented as the means ± SEMs; n = number of observations. (∗) p<0.01, comparing the trained group versus the control group.
Figure 1 demonstrates the increased coronary blood flow that was observed in the swimming-trained group compared with that of the control group (C: 2.46±0.33 vs. T: 5.45±1.47 mL.min-1.g-1; p<0.05).
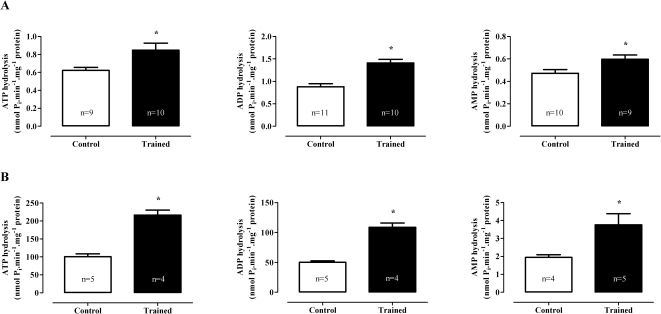
Hydrolysis of Adenine Nucleotides in Blood Serum and Cardiac Sarcolemmal FractionSwimming training increased the hydrolysis of adenine nucleotides in the blood serum fraction as follows (Figure 2A): ATP (C: 0.62±0.03 vs. T: 0.85±0.08 nmol Pi.min-1.mg-1 protein; p<0.05), ADP (C: 0.88±0.07 vs. T: 1.41±0.08 nmol Pi. min-1.mg-1 protein; p<0.001), and AMP (C: 0.47±0.03 vs. T: 0.60±0.04 nmol Pi.min-1.mg-1 protein; p<0.05). Similarly, the hydrolysis of nucleotides in the cardiac sarcolemmal fraction (Figure 2B) were increased by swimming training as follows: ATP (C: 101±8 vs. T: 217±14 nmol Pi.min-1.mg-1 protein; p<0.001), ADP (C: 50±2 vs. T: 109±7 nmol Pi.min-1.mg-1 protein; p<0.001), and AMP (C: 1.9±0.2 vs. T: 3.8±0.6 nmol Pi.min-1.mg-1 protein; p<0.05).
Activity of ATP diphosphoydrolase and 5′-nucleotidase enzymes (nmol Pi.min-1.mg-1 protein) on the blood serum fraction (A) and cardiac sarcolemmal fraction (B). The values are represented as the means ± SEMs. (∗) p<0.05 compared with the control group. The number of animals (n) is indicated in the figure. All of the samples were assayed in duplicate, and only samples in which the two absorbance values were in accordance were considered in the experiment.
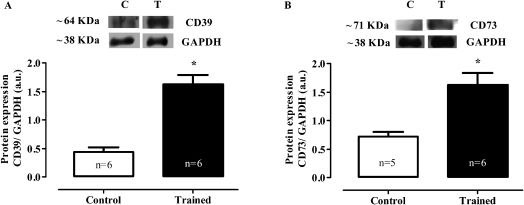
In the heart muscle, the swimming training increased the protein expression of both ectonucleoside triphosphate diphosphohydrolase (protein expression CD39: C 0.44±0.1 vs. T 1.63±0.2 AU; p<0.001) and ecto-5′-nucleotidase (protein expression CD73: C 0.72±0.1 vs. T 1.62±0.2 AU; p<0.01) (Figures 3A and B, respectively).
Representative western blot and densitometric analyses of the heart homogenates (AU). CD39 (A) and CD73 (B) protein expression. The expression of GAPDH is shown as a loading control. The values are represented as the means ± SEMs. (∗) p<0.001 compared with the control group. The number of animals (n) is indicated in the figure.
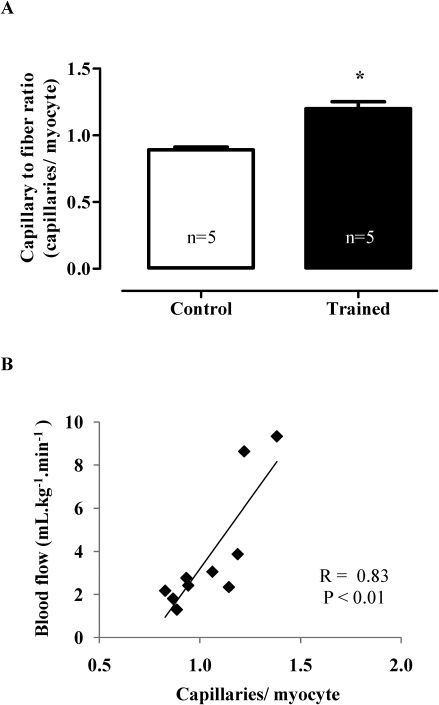
Figure 4A demonstrates that the swimming training induced angiogenesis by the capillary-to-fiber ratio increased (C 0.89±0.02 vs. T 1.20±0.05 capillaries/myocyte; p<0.001). Additionally, Figure 4B shows a high positive correlation between the capillary/myocyte ratio and coronary blood flow (r = 0.83; p<0.01).
A: Capillary-to-fiber ratio. The values are represented as the means ± SEMs. (∗) p<0.001 compared with the control group. The number of animals (n) is indicated in the figure. B: The increase of cardiac capillaries was positively correlated with increased coronary blood flow (control group, n = 5; trained group, n = 5) (r = 0.83, p<0.01).
The primary findings reported here show that swimming training increases coronary blood flow, capillary supply in the myocardial and extracellular nucleotide hydrolysis. This increased nucleotides hydrolysis produces more adenosine, a potent vasodilator that may contribute to augmented coronary blood flow and angiogenesis. Moreover, the exercise-training-induced angiogenic process that was observed in the heart contributes to improved cardiac perfusion.
Regular exercise improves the autonomic control of cardiovascular function and results in the adaptation of the autonomic nervous system, which is commonly observed as a reduced resting heart rate24 and is considered to be a physiological marker for the aerobic adaptation to exercise training.1,2 Indeed, resting bradycardia was observed in the trained group, which confirmed the effectiveness of the exercise training protocol; however, blood pressure did not change as an effect of exercise training. The effect of aerobic exercise training on the blood pressure of normotensive animals and humans seems to be minimal, and our results are consistent with other studies.1,2,25,26
In contrast to the pathological left ventricular hypertrophy that was observed in hypertensive heart disease,27 chronic endurance exercise led to physiological left ventricular hypertrophy3 and coronary vascular adaptations that can improve the myocardial oxygen supply.28 Oxygen supply can also be improved by increased blood flow; in this study, we observed that swimming training increased coronary blood flow (Figure 1), which was previously demonstrated in dogs that had performed a single session of treadmill exercise29 and during hypoxemic conditions that were designed to promote coronary dilatation in rats that underwent treadmill training.30
The alteration in myocardial blood flow may have resulted from the production of adenosine, which is a purine nucleoside that is recognized as a major local tissue-function regulator. Nucleoside production is increased in conditions in which ATP utilization increases, such as during shear stress, hypoxia, and stretching. In the cardiovascular system, the nucleotide hydrolysis chain represents the major route for the generation of extracellular adenosine.9 This study describes for the first time that in the myocardium of normotensive rats that were submitted to chronic swimming training, the activity (Figure 2) and expression (Figure 3) of nucleotidases, such as E-NTPDase (expression of CD39) and 5′-nucleotidase (CD73), were higher than in sedentary rats; this increased the hydrolysis of extracellular nucleotides and contributed to adenosine production.
E-NTPDase 1/CD39 is one of the most studied members of the E-NTPDase family, and it has the same preference for ATP and ADP hydrolysis (1:1). This enzyme has a well-described role in regulating blood flow and platelets by converting ATP and ADP, which are the vasoconstrictor and promoter of platelet aggregation, respectively, to AMP. The association of this enzyme with 5′-nucleotidase/CD-73 converts AMP into adenosine, which is a potent anti-platelet vasodilator. Indeed, the phenotype of the CD39-null mouse is consistent with thromboregulation disorders, which display heightened susceptibility to inflammatory vascular reactions.9,31 Although we only evaluated E-NTPDase 1 (CD39) expression in the myocardium, we cannot rule out the participation of other E-NTPDases that are involved in extracellular nucleotide hydrolysis, primarily E-NTPDase 2 (CD39L1) and E-NTPDase 6 (CD39L2), which are highly expressed in the heart.32 Moreover, circulating nucleotides are also known to be important signaling molecules. Therefore, in this work, we evaluated nucleotide hydrolysis in blood serum. Similar increases were observed in the activity of both soluble enzymes. Therefore, circulating blood serum enzymes, such as E-NTPDases and 5′-nucleotidase, may contribute to the reduction of excess nucleotides and the increase in the concentration of adenosine.
It has been well documented that functional and structural adaptive changes, such as angiogenesis, are induced by chronic exercise training.33 Our results demonstrated an angiogenic process in response to swimming training (Figure 4A). An increased capillary supply in the myocardium of exercised rats has already been described by other researchers.5,34 Regular exposure to the increased shear stress that results from increased blood flow during exercise is considered to be the primary signal for exercise-training-induced adaptations.35 Indeed, we demonstrated a positive correlation between increased coronary blood flow and the number of cardiac capillaries (Figure 4B). One of the effects of adenosine is the promotion of vessel growth, and evidence has indicated that adenosine plays an important role in neovascularization (including angiogenesis and vasculogenesis).12 Higher levels of adenosine may be an important factor for the angiogenesis that is induced by exercise training due to adenosine's capacity to increase levels of pro-angiogenic molecules (e.g., VEGF). Studies in humans and animals have confirmed myocardial capillary proliferation in response to exogenous adenosine or augmented endogenous adenosine.12 Therefore, enhanced angiogenesis is an adaptive response to functional adaptations, such as augmented local blood flow and higher adenosine levels, which are induced by exercise, and angiogenesis is a prominent beneficial effect of exercise training on the myocardium.
The present study demonstrated that chronic swimming training is efficient for producing functional and structural adaptations of the cardiovascular system. Changes in the extracellular hydrolysis of adenine nucleotides by augmented activity and the expression of enzymes, such as E-NTPDase and 5′-nucleotidase, may increase adenosine production and could be among the mechanisms that contribute to the increased coronary blood flow and angiogenesis that is observed in exercise-trained rats.
FR Roque was the recipient of a FAPESP scholarship (no. 05/52100-2), and UPR Soci was the recipient of a CNPq-PIBIC scholarship. EM Oliveira and MC Irigoyen hold scholarships from CNPq, Brazil.
No potential conflict of interest was reported.
Roque FR is the principal investigator of the study, obtained a student scholarship from FAPESP for project development, and participated in all stages of the study. Soci UPR assisted in the training of animals and in the experimental protocol for the cardiac capillaries. De Angelis K performed the protocol for the investigation of coronary blood flow (colored microspheres). Coelho MA performed the surgical process on the animals. Furstenau CR assisted in the western blot experiments. Vassallo DV assisted in the discussion of the results and in the manuscript preparation. Irigoyen MCC is responsible for the Laboratory of Hypertension, where the hemodynamic and coronary blood flow experiments were performed and provided all the necessary materials and equipment for the experiments. Oliveira EM is the investigator responsible for the project, and is the one who obtained scholarships from CNPq.