Cerebral palsy is the most common cause of physical disability in children. Spasticity is a disabling clinical symptom that is prevalent among patients suffering from cerebral palsy. The treatment of spasticity with botulinum toxin type A (BTX-A) is a well-established option in the interdisciplinary management of spasticity, providing focal reductions in muscle tone in cerebral palsy patients.
OBJECTIVE:The aim of this retrospective study was to describe the effect of multilevel BTX-A injections in the lower extremities, focusing mainly on gross motor function and functional status in cerebral palsy patients.
METHODS:Data from 71 cerebral palsy patients (64% male, 36% female, mean age 6.7 ±3.2 years) were analyzed retrospectively. We used the Ashworth and Tardieu scales to evaluate the degree of spasticity. Motor function was measured by the Gross Motor Function Measure (GMFM–88), and functional status was classified by the Gross Motor Function Classification System (GMFCS I-V). Multilevel BTX-A injections were applied after sedation and with electrostimulation guidance. The evaluations were repeated every three months, and the patients were followed for six months.
RESULTS:We found that the Ashworth and Tardieu scores decreased significantly at the three-month evaluation (p<0.05) but not at the six-month evaluation (p>0.05). Although the improvement in spasticity was not maintained at the six-month evaluation, GMFM-88 scores increased significantly at the three- and six-month assessments. GMFSC levels showed no change in the three- and six-month assessments.
CONCLUSION:We believe that a single multilevel BTX-A injection reduces spasticity and improves motor function in children with cerebral palsy.
Cerebral palsy (CP) is the most common cause of physical disability in children, with an incidence of 1.5–3 cases per 1000 live births.1–3 Spasticity is a disabling clinical symptom that is prevalent in those with CP.2 The primary problems associated with spasticity are the loss of balance, strength and selective motor control of the muscles, as well as increased muscle tone, leading to secondary problems such as fixed contractures and bony deformities, causing severe motor dysfunction3–6 in patients. Treatment with botulinum toxin type A (BTX-A) has been a well-established option in the interdisciplinary management of spasticity since the late 1980s, providing focal reductions in muscle tone in CP patients.2 However, interpretations of the literature regarding this subject are challenging because of the difficulties of measuring spasticity and functional changes in children.
Defining the goals of CP treatment is one of the main issues in its management. These goals, which should be individualized for each patient, are to reduce muscle tone, increase the range of motion of the joints, improve the function of upper and lower extremities, delay the need for surgery, and predict surgical results.3,7 BTX-A injections should always be considered as an adjunctive treatment to traditional therapies, including physiotherapy, orthoses, occupational therapy, and serial casting.3,8
Several randomized and non-randomized controlled studies have demonstrated the effectiveness of BTX-A injections in reducing muscle tone, increasing the range of motion, and improving posture and gait in CP patients.8–11 Nevertheless, some studies have demonstrated little benefit from BTX-A with respect to functional gains and quality of life.7 Although this discrepancy between the various studies may be explained by differences in method, this point requires further elucidation.
The aim of this retrospective study was to describe our experience regarding the effect of multilevel BTX-A injections in the lower extremities, mainly pertaining to gross motor function and functional status in CP patients.
METHODSParticipantsData from 131 children with CP who were treated with BTX-A from 2003 to 2008 were investigated for inclusion in this retrospective study. Records were selected from patients with spastic CP (hemiplegia, diplegia or quadriplegia), well-documented clinical histories and appropriate physiotherapy and orthoses for day and night use. Records were excluded for patients with these conditions: spasticity predominantly in their upper extremities, fixed contractures and hip, knee and ankle deformities; or a history of previous surgery or application of intrathecal baclofen. A deficit in cognitive function was not a criterion for exclusion. Patients who were received more than one injection session and those with records lacking sufficient data were also not included. Of the 131 patients evaluated, the records of 71 (64% male, 36% female, mean age 6.7 ±3.2 years) who received one BTX-A injection were selected. Informed consent was obtained from the children’s parents before the procedure.
ProcedureCP patients were referred to the Physical Medicine and Rehabilitation (PM&R) clinic after being evaluated by the Pediatric Neurology clinic and were examined and documented by a PM&R specialist. Following this recording procedure, pediatric neurology specialists and orthopedic surgeons re-evaluated the patients and individualized therapeutic goals were planned. The children’s motor functions were measured by the Gross Motor Function Measure (GMFM–88)13. GMFM is a criterion reference tool that is designed to measure changes in gross motor function over time in children with motor impairment and has been validated for sensitivity to changes in children with CP. Functional status was classified by GMFCS I-V. GMFCS for CP is based on self-initiated movement, which represents the child’s present abilities and limitations in motor function.14 Spasticity was assessed using the Ashworth and Tardieu scales.15, 16 The assessments were repeated at the three- and six-month points following the BTX-A injection.
InterventionThe injections were administered using a multilevel approach at a single injection session. The various muscle groups that were injected in each session were the gastrocnemius, medial and lateral hamstring muscles, the adductors, iliopsoas, tibialis posterior, soleus and rectus femoris. Of the 131 children assessed, 71 had received one BTX-A session with sufficient follow-up data. A standard concentration of BTX-A (Botox 100 U/ml or Dysport 500 U/ml) was used. The toxin dosage was calculated using a dose conversion ratio of 5:1 U for Dysport: Botox, which was shown to be similar in clinical efficacy between two formulations.5 The dosage for Botox (Allergan, USA) ranged from 15–20 U/kg, and that for Dysport (Ipsen, UK) was 30 U/kg. If only one side was injected, the dose was 15 U/kg. The total maximum doses for Botox and Dysport were 300 U and 500 U, respectively. The total muscle dose was divided between 2–4 injection sites, depending on muscle size. The injections were administered under electromyography (EMG) or electrical stimulation guidance for the exact identification of target muscles and motor points. Special monopolar needle electrodes (Myoject) were used for the injections. Oral sedation with midazolam 0.5 mg/kg was administered 30 minutes before administration of the injections.7
Physical Therapy ProgramThe patients underwent regular physiotherapy sessions before the BTX-A injections. An intensive physiotherapy program was started one week after the multilevel BTX-A injections. The first 3 weeks consisted of 45 min/day, 5 days/week, after which the level was decreased to 3 days/week for an additional 8 weeks. The physiotherapy sessions consisted of active and passive stretching of flexor muscles, strengthening of extensor muscles, and balance and gait training.
OrthosesAnkle foot orthoses were used to support full knee extension and to correct plantar flexion deformity of the ankle. Night splints were prescribed to maintain the neutral position of the ankle and to ensure sufficient stretching of the plantar flexor muscles.
Follow-upThe patients were assessed at baseline and every three months post-injection for six months.
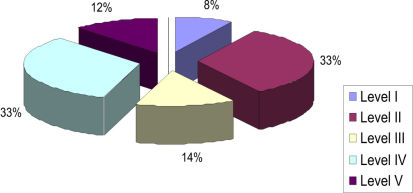
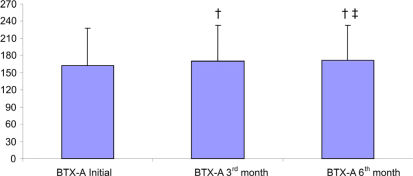
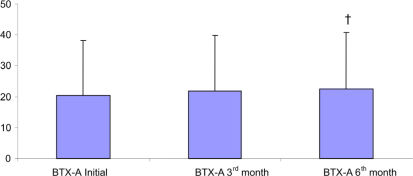
RESULTSData from the 71 patients who were followed for six months were evaluated. The demographic data are presented in Table 1. The distribution of patients according to GMFCS level is shown in Figure 1. The improvements in Ashworth scores and Tardieu test findings are shown in Tables 2 and 3. Total GMFM–88 scores are shown in Table 4. A comparison of the initial total GMFM-88 scores with those at the three- and six-month evaluations is shown in Figure 2. The GMFM (lying and rolling) and GMFM (crawling and kneeling) scores improved significantly at the six-month evaluation compared with the initial scores (p<0.05 and p<0.01, respectively). The GMFM (sitting) and GMFM (standing) scores improved significantly at the three- and six-month evaluations (p<0.05 and p<0.001 (sitting); p<0.001 (standing) for both, respectively). The improvements in GMFM (walking, running and jumping) scores are shown in Figure 3. The GMFCS scores showed no significant improvements at any assessment (p>0.05).
Tardieu scores.
| MUSCLES | Initial Mean ±SD | Third month Mean ±SD | Sixth month Mean ±SD |
|---|---|---|---|
| Hip Adductor Muscles | |||
| Right | 1.36 ±1.14 | 1.30 ±1.16 | 1.32 ±1.06 |
| Left | 1.39 ±1.13 | 1.35 ±1.16 | 1.38 ±1.04 |
| Hamstring Muscles | |||
| Right | 1.56 ±1.05 | 1.45 ±1.10 | 1.57 ±0.93 |
| Left | 1.60 ±1.03 | 1.50 ±1.08 | 1.57 ±0.93 |
| Gastrosoleus Muscles | |||
| Right | 2.18 ±1.11 ¥ | 1.95 ±1.00 | 2.04 ±1.02 |
| Left | 2.18 ±1.20 ¥ | 2.02 ±1.15 † | 2.16 ±1.10 |
Ashworth scores.
| MUSCLES | Initial Mean ±SD | Third month Mean ±SD | Sixth month Mean ±SD |
|---|---|---|---|
| Hip Flexor Muscles | |||
| Right | 0.98 ±1.05 ¥ | 0.65 ±0.79 | 0.87 ±0.96 |
| Left | 1.01 ±1.09 ¥ | 0.68 ±0.80 | 0.94 ±0.99 |
| Hip Adductor Muscles | |||
| Right | 1.61 ±1.47 ¥ | 1.25 ±1.25 | 1.47 ±1.36 |
| Left | 1.74 ±1.51 ¥ | 1.32 ±1.25 † | 1.58 ±1.39 |
| Hamstring Muscles | |||
| Right | 2.10 ±1.55 * | 1.58 ±1.33 † | 2.08 ±1.48 |
| Left | 2.18 ±1.54 ¥ | 1.65 ±1.33 † | 2.10 ±1.48 |
| Gastrosoleus Muscles | |||
| Right | 2.61 ±1.21 * | 1.90 ±1.12 £ | 2.53 ±1.25 |
| Left | 2.70 ±1.25 * | 1.97 ±1.25 £ | 2.64 ±1.27 |
Scores of the Five Dimensions of GMFM–88.
| GMFM | Initial Mean ±SD | Third month Mean ±SD | Sixth month Mean ±SD |
|---|---|---|---|
| Total | 162.35 ±65.48 *¶ | 169.85 ±63.61 † | 171.47 ±61.86 |
| Lying and Rolling | 25.09 ±3.17 § | 25.74 ±2.56 | 25.87 ±2.46 |
| Crawling and Kneeling | 22.59 ±3.40 §¥ | 23.08 ±2.96 | 23.16 ±2.93 |
| Sitting | 47.90 ±17.18 ¥¶ | 49.19 ±16.52 | 49.87 ±15.70 |
| Standing | 15.00 ±13.50 *¶ | 16.64 ±13.71 | 17.00 ±13.91 |
| Walking, Running, Jumping | 20.30 ±17.79 *¶ | 21.77 ±18.08 | 22.45 ±18.35 |
GMFM: Gross motor function measurement scale. BTX-A: Botulinum toxin type A.
This retrospective study evaluated the effect of multilevel BTX-A injections and intensive physiotherapy on motor function and spasticity in children with CP six months after injection. The results showed that multilevel injections of the muscles of the lower extremities significantly reduced muscle tone. More importantly, these injections also resulted in improvements in patients’ motor functions.
When injected into the muscle, BTX-A produces local muscle weakness by blocking the release of acetylcholine at the neuromuscular junction. The toxin’s effect on motor function is observed within a few days following injection and lasts between three and four months, a period related to the time required for new synaptic connections to occur with collateral sprouting17. Several studies have proved the long-term safety and efficacy of this treatment. A meta-analysis of double-blind, randomized controlled clinical trials showed the superior effectiveness of BTX-A over a placebo on the improvement of gait in patients with spastic equinus foot.18 The decreased spasticity allows better stretching of the agonist muscles, increases the range of motion of the joints and strengthens the antagonist muscles. Although the physiological effect of the toxin seems to be reversible, its use helps children learn to control their movements and improve function.5,19,20
We found that the muscle tone of the hip flexors, adductors, knee flexors and ankle plantar flexors was significantly reduced at the three-month assessment. However, this effect on spasticity did not last until the end of the sixth months because muscle tone increased between the three- and six-month evaluations. These findings are compatible with various recent studies. Scholtes et al.,8 in their randomized controlled study, demonstrated the effectiveness of BTX-A on muscle length, spasticity and gait parameters. They observed a reduction in spasticity beginning six weeks after multilevel injections and showed that the lack of this effect at 24 weeks post-injection supported the temporary effect of the toxin due to the restoration of neuromuscular junctions. A controlled pilot study19 evaluated the effect of BTX-A injection on spastic equinus foot in CP patients and showed a significant decrease in Ashworth scale scores and an increase in the range of motion of the ankle joint at the end of the first month, which was supported by both pedobarometry and surface EMG findings.21
Despite the temporary effect of BTX-A, the effect on motor functions could last for a longer period of time. In our study, we determined an increase in the total GMFM-88 score and various dimension scores (lying and rolling, crawling and kneeling, sitting, standing, walking-running-jumping) at the three-month evaluation, an effect that did not disappear at the six-month evaluation. Despite these findings, there was no significant difference in GMFCS I-V. We believe that the change in motor abilities is better reflected by the GMFM-88 score and its five dimensions. GMFCS classifies the ordinary performance of the patient, not the quality of the present gross motor function. In one of our previous studies involving 18 CP patients, we demonstrated reduced spasticity, increased range of motion and improved GMFM–88 scores five weeks after multilevel BTX injections. Although the range of motion and spasticity findings were similar to their initial values at the 12th week post-injection, the improvements in GMFM–88 scores were still significant. However, in that study, we did not use the GMFCS for the classification of ambulatory status of the patients.9 Ross et al.22 evaluated the relationship between spasticity, strength and functional measures in a retrospective study and found that the GMFM scores did not correlate with spasticity. Their results showed little or no significant relationship between spasticity and function. This finding might help to explain our observation at the six-month post-injection assessment, as the patients seemed to be functionally better despite the fact that their spasticity had almost returned to baseline levels. Bjornson et al.,2 in their randomized placebo-controlled study, documented functional improvement by means of increased GMFM–66 and GMFM–88 scores at the sixth month post-injection. They explained this finding as reflecting the adjustment to the changes in motor strength, range of motion and spasticity. This finding was supported by a study by Scholtes et al.,23 which reported improvements in GMFM–66 scores at the sixth month after multilevel injections of CP patients. Slawek et al.11 reported that BTX-A reduced spasticity, increased the range of motion of the ankle joint and increased motor functions in diplegic CP patients who were followed for three months post-injection. Although there was an increase in spasticity at the end of the third month, the GMFM scores did not return to baseline levels. El et al.12 treated 14 diplegic CP patients with BTX-A injections and then followed them for six months. They observed improvements in clinical parameters, such as spasticity and motor function, during the first month post-injection. Although there was an increase in spasticity at the six-month evaluation, they also observed an improvement in motor function (GMFCS score). In our study, we observed significant improvements in GMFM-88 scores but not in GMFCS scores at the end of the sixth month. In contrast to the study of El et al., which included only diplegic CP patients, 26% of the patients in our study group were quadriplegic, and 47% were diplegic. Thirty-three percent of our patients were GMFCS level IV, and 33% were level V; thus, the majority of our study group consisted of severely affected patients. This methodological difference might help to explain our results. However, Weigl et al.7 followed their patients with GMFCS and used a goal attainment scale (GAS). They found no significant improvements in GMFCS scores and concluded that BTX-A treatment did not maintain improvements in motor functions. They did not use GMFM–88 in their study to evaluate gross motor functions, and we believe that this evaluation is necessary to make such a claim. Nevertheless, their findings are similar to ours.
Multilevel BTX-A injections for the treatment of spasticity in CP, have been widely accepted by many researchers for more than 15 years.3 They have been shown to achieve better results than single-level treatments in children with CP.24 The BTX-A dosage used varied in different studies, ranging from 10 U/kg to 25 U/kg for Botox and 30 U/kg for Dysport. Willis et al.25 used higher doses of Botox (20–25 U/kg) in a controlled study and reported them to be safe. They mentioned that both high and low doses result in reduced spasticity and an improvement in the range of motion. In a recently published study, dose of 7.45±2.06 U/kg Botox, produced improvements in gait patterns and increased the range of motion of the ankle in patients with spastic diplegic CP.26 In our study, we used 15–20 U/kg Botox and 30 U/kg Dysport, which can be regarded as moderate dosages.
BTX-A injections should always be used as an adjunctive treatment to physiotherapy, occupational therapy and orthotic management. It is well-accepted that a combined approach of pre- and post-injection physiotherapy provides long-term benefits.3,5,17 Stretching of the flexor muscles, strengthening of the extensor muscles and exercises to improve gait should be intensively applied, especially in the first weeks, when the toxin is more pharmacologically active. Appropriate orthoses should be planned for joint stabilization during ambulation and to maintain muscle length.8 The patients in our study group received at least six months of pre-injection physiotherapy and continued to be treated with appropriate conservative treatments consisting of intensive physiotherapy (five times/week) for the first three weeks and a reduced regimen (three times/week) in the following 8 weeks. Although this study is a retrospective study without a control group, which is one of its recognized limitations, we observed the effectiveness of a conservative approach in patients treated with BTX-A.
In our study, the mean patient age was six years. It is believed that better results may be obtained in patients who are treated at younger ages. As children grow, the risk of fixed contractures and bony deformities increases.3 Although our study is not an age-matched controlled study, we believe that our results support the idea that younger children may receive more benefits from multilevel BTX-A injections, intensive physiotherapy and appropriate orthotic management compared to older children.
In conclusion, we believe that optimally timed, multilevel BTX-A injections are effective in reducing spasticity and maintaining functional gains at six months post-injection. We suggest that the evaluation tools for this effect should involve more detailed scales, such as GMFM–88, to properly demonstrate functional gains. The importance of physiotherapy and appropriate orthoses should always be mentioned to improve the efficacy of the toxin in maintaining improvement in strength, selective motor control, balance and gait.