To determine the safety and efficacy of a transdermal nanostructured formulation of progesterone (10%) combined with estriol (0.1%) + estradiol (0.25%) for relieving postmenopausal symptoms.
METHODS:A total of 66 postmenopausal Brazilian women with climacteric symptoms of natural menopause received transdermal nanostructured formulations of progesterone and estrogens in the forearm daily for 60 months to mimic the normal ovarian secretory pattern. Confocal Raman spectroscopy of hormones in skin layers was performed. Clinical parameters, serum concentrations of estradiol and follicle-stimulating hormone, blood pressure, BI-RADS classification from bilateral mammography, and symptomatic relief were compared between baseline and 60 months post-treatment. Clinicaltrials.gov: NCT02033512.
RESULTS:An improvement in climacteric symptoms was reported in 92.5% of women evaluated before and after 60 months of treatment. The serum concentrations of estradiol and follicle-stimulating hormone changed significantly (p<0.05) after treatment; the values of serum follicle-stimulating hormone decreased after 60 months from 82.04±4.9 to 57.12±4.1 IU/mL. A bilateral mammography assessment of the breasts revealed normal results in all women. No adverse health-related events were attributed to this hormone replacement therapy protocol.
CONCLUSION:The nanostructured formulation is safe and effective in re-establishing optimal serum levels of estradiol and follicle-stimulating hormone and relieving the symptoms of menopause. This transdermal hormone replacement therapy may alleviate climacteric symptoms in postmenopausal women.
There is an ongoing debate about the side effects of drugs administered orally (1-2). First-pass metabolism is associated with many side effects, as the metabolites of these compounds affect human physiology (3).
Conventional hormone therapy delivered by the oral route is used to relieve menopausal symptoms. Transdermal estrogens have not been used extensively in Brazilian women with menopausal symptoms; however, a recent long-term study indicated that there is no increased risk of breast cancer or vasomotor disorders in menopausal women using transdermal hormone replacement therapy (HRT) (3).
The technology of controlled-released drugs represents a innovative field and this kind of strategy may provide important contribution for human health (4,5). Recent studies on this methodology have used confocal Raman spectroscopy to describe in real time the drug concentration in each layer of the skin (3,4).
Nanotechnology is a potent and effective tool that brings new perspectives to the health sciences, such as transdermal absorption. Recently, new and strong evidence has suggested that these types of drugs have interesting and unique properties (5,6).
The menopausal period is characterized by a significant decrease in estrogen and progesterone production and by an increasing follicle-stimulating hormone (FSH) levels (7). Hormone replacement therapy (HRT) with exogenous hormones relieves climacteric symptoms, but there are few long-term studies focused on transdermal hormone replacement therapy (THRT) as a strategy for treating climacteric symptoms (8).
HRT may have protective effects on osteoporosis (9) and heart disease (10). In recent studies, this hypothesis has been questioned because long-term use has fewer benefits than short-term use [11-14]. A recent long-term study on THRT yielded interesting findings regarding the safety and efficacy of an enhancer capable of delivering nanoparticles containing estriol and estradiol through the skin in menopausal women (3).
The present study was designed to determine the long-term effects of transdermal HRT using a nanostructured formulation of estriol (0.1%) combined with estradiol (0.25%) and progesterone (10%) to treat menopausal symptoms. The therapeutic effects were evaluated by clinical, laboratory, and radiographic parameters after a 5-year follow-up.
SUBJECTS AND METHODSEthicsInitially, written informed consent was provided by individuals willing to participate in a protocol approved by the ethics committee of University Paulista, UNIP, São Paulo, Brazil. Written informed consent was obtained from every subject (UNIP #533/2009). The study was registered at Clinicaltrials.gov: NCT02033512.
Study protocolThis prospective long-term clinical trial included female patients aged 51-70 years who were treated for menopause-related hormone imbalances. Other results from this study are published elsewhere. Volunteers were recruited from the Gynecological Medical Center in Fortaleza, Ceará, Brazil, where patient charts are maintained.
Study populationThis prospective long-term clinical trial assessed the effects of transdermal HRT on climacteric symptoms and hormone serum levels in early postmenopausal women. Preliminary results from the first 42 women enrolled have been previously published (3).
The 91 women enrolled in this trial fulfilled the following inclusion criteria: 1) last menstrual period between 6 months and 3 years before the beginning of the study and follicle-stimulating hormone (FSH) levels higher than 35 IU/L; 2) age between 51 and 70 years; and 3) no use of any medication known to interfere with hormonal levels in the past 6 months. Patients presenting with diabetes, previous hysterectomy, endometrial thickness greater than 5 mm, history of cancer, thromboembolism, or established cardiovascular disease were excluded.
Approximately 30% of the initial population was excluded from the study. The most common reason for exclusion was lack of the minimal number of consultations required. During the study, 25 participants were non-compliant with the treatment protocol and were excluded. The final studied population consisted of 66 postmenopausal women who were included in the transdermal hormone therapy protocol and the final data analysis.
In the present study, the subjects received a transdermal nanostructured formulation of estriol (0.1%) + estradiol (0.25%) and progesterone (10%) (Biolipid B2®, Evidence, SP, Brazil) in the right and left forearms, respectively, daily for 60 months. The effects of transdermal hormone treatments were analyzed.
Clinical evaluations were performed before the treatment. Anthropometric measurements included body weight and blood pressure, and bilateral mammographies were performed before and during treatment. Blood pressure was measured using a digital sphygmomanometer (Omron HEM 742, Rio de Janeiro, Brazil). All radiographic exams were performed by experienced, board-certified radiologists.
Nanostructured emulsion preparationThe nanoemulsion was developed at the Laboratory of Nanotechnology at University Potiguar in association with the Laboratory of Advanced Materials at Federal University of Ceará. Two hormone + BIOLIPID/B2® formulations were prepared, and the following mass ratios were obtained: nanoformulation 1: estriol (0.1%) + estradiol (0.25%) + Biolipid-B2®; and nanoformulation 2: progesterone (10%) + Biolipid-B2®.
Progesterone, estriol, and estradiol were purchased from Sigma Aldrich (Saint Louis, MO, USA). The main components of the emulsion were the nanoparticulated hormones and a transdermal penetration enhancer vehicle (Biolipid® B2, Evidence Pharmaceuticals, São Paulo, SP, Brazil) containing oleic acid, phospholipids, and nutrients compatible with the dermal structure, which enhances transdermal drug delivery (3).
The hormone nanoparticles were prepared using a water-oil emulsion method with slight modifications (PCT Patent #WO 2012/009778 A2). The hormone nanoparticles were emulsified using a homogenizer (Art Labortechnik, Müllheim, Germany) at 20,000 rpm for 180 s. The resultant emulsions were stored for 3 h at room temperature (3).
Size and zeta-potential measurementsThe size and zeta-potential of the progesterone particles were measured by a Zetasizer Nanoseries-ZS90 (Malvern, UK). The size measurements were performed in disposable sizing cuvettes at a laser wavelength of 633 nm and a scattering angle of 90°, while the zeta-potential measurements were performed in disposable zeta-potential cells. Before the measurements, the progesterone particles were diluted 1:360 in Milli-Q water. Each measurement was repeated for 3 runs per sample at 25°C.
Particle size measurementsParticle size analysis was performed by dynamic light scattering (DLS), also known as photon correlation spectroscopy, using a particle size analyzer (Zetasizer Nanoseries-ZS90, Malvern, UK). Prior to the measurements, all samples were diluted (1:360) using Milli-Q water to yield a suitable scattering intensity. DLS data were analyzed at 25°C and with a fixed light incidence angle of 90°. The mean hydrodynamic diameter (Z-average) and the polydispersity index (PDI) were determined as a measure of the width of the particle size distribution. The Z-average and PDI of the analyzed samples were obtained by calculating the average of 13 runs. The measurements were performed in triplicate.
Clinical evaluationThe THRT consultation consisted of a brief lecture about the risks and benefits of transdermal HRT. During the entire treatment period, patients were asked to respond to questions about adverse effects using a standardized form to monitor symptom resolution. The patients were questioned regarding climacteric symptoms according to the Kupperman index. During this evaluation, a standardized form was used to monitor symptom resolution and adverse effects.
Blood samples were collected from the subjects early in the morning after an overnight fast. After serum testing, hormone deficiencies were identified, and if necessary, additional transdermal progesterone was prescribed. The patients were evaluated 3 months after completion of the THRT protocol.
To guarantee standardization and correct use of the THRT, all patients were instructed on how to use the progesterone pump for transdermal application by an experienced physician. Compliance was defined as completing 70% or more of the transdermal applications.
Compliance and satisfaction with the treatment were also evaluated by personal interviews. Furthermore, serum concentrations of blood lipids and biochemical inflammatory markers were measured, and the results are published elsewhere.
During the 60-month trial, patients were instructed to complete evaluation forms every 3 months after the first consultation to monitor symptoms and side effects. In addition, demographic data, including age and level of education, were assessed.
Transdermal hormone replacement therapy (THRT) assayOn the right forearm, patients received a transdermal dose (1 pump of 0.8 g) of progesterone (10%) + Biolipid®B2, and on the left forearm, they received another pump (0.8 g) of estriol (0.1%) + estradiol (0.25%) + Biolipid®B2. This nanoformulation was administered daily for 60 months.
The severity of postmenopausal symptoms was evaluated by the form submitted after each 6 months of the study. Satisfaction with the transdermal hormone therapy was also evaluated at those times. Compliance with the regimen was checked by personal interviews. Furthermore, blood pressure and serum concentrations of follicle-stimulating hormone (FSH) and estradiol were measured. During the 5-year trial, patients were instructed to complete evaluation forms every 6 months after the first consultation to monitor postmenopausal symptoms and side effects. A clinical examination with the same parameters was repeated after 3 months and every 6 months thereafter during the 5-year period.
Serum level assaysThe patients underwent a baseline blood test before initiating the THRT. Samples were analyzed at the same clinical analysis laboratory, where the personnel were blinded to treatment status.
Serum levels of FSH were obtained by radio-immunoassay (Amerlite FSH). The FSH results were expressed as units per liter (IU/L). Blood was collected from each participant at the baseline visit, 3 months after treatment initiation, and every 6 months for 60 months. Serum levels of estradiol were obtained by radio-immunoassay. The estradiol results were expressed as picograms per milliliter (pg/mL).
Raman spectrometer assayRaman confocal spectroscopy measurements were performed using the model 3510 Skin Composition Analyzer (River Diagnostics, Rotterdam, Netherlands). The test area (4×4 cm) was marked on the forearm of each volunteer. The area was treated with 70 μL of the estriol (0.1%), estradiol (0.25%), and progesterone (10%) formulation and evaluated. The formulation was applied to the skin and gently spread, without rubbing, using the tip of the micropipette. After 10 minutes, the measurements were initiated.
The forearm of the volunteer was placed on a fused silica window adapted to the measurement stage. Laser light was focused on the skin with a microscope objective located under the window. An internal video camera enabled inspection of the skin surface and selection of the measurement spot. To confirm the results, the experiments were performed twice on the volar forearms of the volunteers (3,4).
Detailed Raman depth profiles were acquired through the stratum cornum, viable epidermis, and dermis. All Raman spectra were calibrated and corrected for instrument response using built-in instrument control software (River Diagnostics).
Depth profiles were collected 1, 3, 6, 21, and 24 hours after transdermal progesterone application. Six depth profiles were collected at each time point.
BI-RADS evaluationThe criteria adopted for BI-RADS exam classification were based on the American College of Radiology (ACR) guidelines. A negative screening examination is defined as one that is negative or yields benign findings (BI-RADS categories 1 and 2), and a positive screening examination is one that leads to a referral (BI-RADS categories 0, 4, or 5). The mammograms were evaluated independently by 2 certified radiologists who decided whether referral for further diagnostic assessment was necessary.
Scanning electron microscopy (SEM) imagesThe electron microscopy analysis of the nanoparticles was conducted with a TESCAN SEM (Model VEGA/XMU, Brno, Czech Republic) using an accelerating voltage of 30 kV. All samples that were analyzed by SEM were previously sputtered with an approximately 20-nm gold layer.
Statistical analysisAll statistical analyses were performed using the SPSS statistical package for Windows version 10.0 (SPSS Inc., Chicago, IL, USA). The data are presented as the means ± SEMs or as the medians. Differences between baseline and post-treatment mean values were evaluated by Student's t-test. The Friedman and sign tests were used for analysis of the Kupperman score. Categorical variables were compared using the chi-square and Fisher's exact tests. Normally distributed variables were reported as means (standard deviations). Paired data were compared using the Wilcoxon signed-rank test. All tests were two-sided. Significant differences were defined as p<0.05.
RESULTSAlmost 25% of the initial population did not meet the inclusion criteria. Twenty-three patients were excluded from the study due to lack of consultation, viral infection, or urinary tract infection during the study.
Clinical evaluation was performed before the initial treatment and every 6 months during the trial. The anthropometric measurement included in this study was the body weight. The Kupperman score was assessed before and during treatment, and blood pressure was checked twice during each visit.
The effects of transdermal emulsions were beneficial during 60 months of follow-up. Table1 presents the age, weight, blood pressure, and Kupperman score for 66 menopausal patients before and after 60 months of treatment. The changes in weight and systolic and diastolic blood pressure due to THRT were not statistically significant.
Clinical and anthropometric variables and post-menopausal symptoms of women after 0 (baseline) and 60 months of THRT.
| Baseline (n = 91) | After THRT (n = 66) | Significance | |
|---|---|---|---|
| Age (years) | 58.42±5.26 | 63.4±5.27 | |
| Weight (kg) | 63.68±9.01 | 63.20±8.0 | p = 0.32 b |
| Systolic blood pressure (mmHg) | 12.2±1.18 | 11.98±0.95 | p = 0.16 b |
| Diastolic blood pressure (mm Hg) | 7.67±7.9 | 7.60±0.63 | p = 0.41 b |
| Kupperman score | 25 (16-29) | 5 (0-4) | p<0.01 * |
| Undefined BI-RADS | 7 | 4 | p = 0.90c |
| Treatment satisfaction (%) | 75 | 92.5 | p<0.05c |
Values are expressed as the mean ± SD.
p = difference between baseline and completion of the 60-month THRT.
At baseline, all patients presented climacteric symptoms that improved significantly with treatment, as demonstrated by the Kupperman score (Table1). The extent of satisfaction with the transdermal nanostructured hormone therapy at baseline was ∼75%.
With continued treatment, satisfaction increased to 85.2% after 1 year and 92.5±4.2% at the end of the study (Table1). These values were statistically significant (p<0.05) compared with the mean values from baseline measurements.
Adverse eventsNo patients reported vaginal bleeding or any other adverse effects during the study. Ultrasound assessment of the breasts following 60 months of treatment revealed no tumors in any patients.
Effect of progesterone (10%) associated with the estriol (0.1) + estradiol (0.25%) nanoparticle formulation on BI-RADS classificationPrior to the study, 7 patients were diagnosed with BI-RADS classification B0. After 60 months, no patient was diagnosed with BI-RADS 4 or 5. At the end of the treatment, only 4 patients were diagnosed with a B0 classification (Table1).
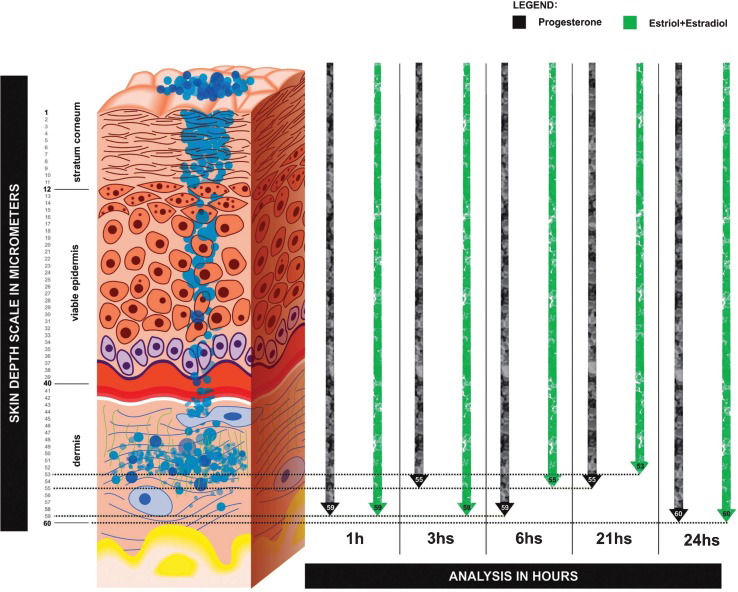
Treatment outcomesDepth and concentration of nanoparticles of progesterone (10%) associated with estriol (0.1%) + estradiol (0.25%) nanoparticle formulation on skin layersFigure1 shows a graphical representation of estriol + estradiol and progesterone nanoparticles flowing through the skin layers. These data were acquired through confocal Raman spectroscopy analysis (CRSA) (River Diagnostics, Skin Composition Analyzer/3510, Rotterdam, Netherlands). These results represent the concentration and depth of nanoparticles in each skin layer at 1, 3, 6, 21, and 24 hours after the transdermal HRT clinical procedure.
Effect of progesterone (10%) associated with the estriol (0.1) + estradiol (0.25%) nanoparticle formulation on estradiol serum levelsThe serum levels of estradiol during the 60 months of THRT are shown in Figure2. The mean estradiol pre-treatment value was 28.88±39.62 (pg/mL), and this value showed a statistically significant increase (p<0.05) after 60 months of THRT to 51.85±77.50. The data revealed a significant difference (p<0.05) after treatment with the transdermal formulation (Figure2).
Effect of the transdermal nanoemulsion on estradiol serum levels (ESLs) in 66 women receiving transdermal hormone replacement therapy (THRT) for 60 months. The nanoemulsion was administered topically in postmenopausal women. Mean values are shown, and the SEs are indicated by error bars. *p<0.05 was considered significantly different from baseline values (Student's t-test).
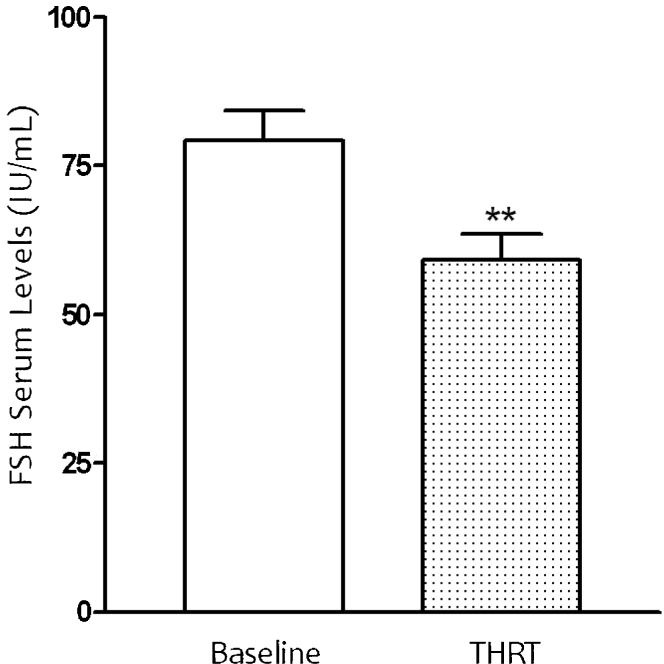
The serum levels of estradiol and FSH were observed during the 60 months of THRT (Figure4a). The mean FSH pre-treatment value was 97.75±45.27 IU/mL, and after 60 months of THRT, this value showed a statistically significant decrease to 63.39±44.14 IU/mL. The data reached a statistical difference (p<0.05) after treatment with the transdermal formulation (Figure3).
A. Zeta potential analysis on Progesterone (10%) formulation administered topically in 66 postmenopausal women submitted to THRT during 60 months. Zetasizer Nano ZS90 (Malvern Instruments Ltd., UK, England). B. Zeta potential analysis on Progesterone (10%) formulation administered topically in 66 postmenopausal women submitted to THRT during 60 months. Zetasizer Nano ZS90 (Malvern Instruments Ltd., UK, England). C. Scanning Electron Microscopic Analyses of progesterone particles morphology administered topically in 66 postmenopausal women submitted to THRT during 60 months (TESCAN SEM-Model VEGA/XMU, Brno, Czech Republic). D. Raman Confocal spectroscopy analysis on skin layers. The skin depth concentration of progesterone was measured at 1, 3, 6, 21 and 24 hours in Stratum Cornum (SC); Viable Epidermis (EPI); Dermis (D) after transdermal application. Mean values are shown, SEM is indicated by error bars. *p<0.05 was considered significantly different compared to the first hour values (Model 3510 SCA, River Diagnostics, Rotterdam, The Netherlands).
Effect of nanoparticle formulation emulsion on FSH serum concentration in 66 women receiving transdermal hormone replacement therapy (THRT) for 60 months. The nanoemulsion was administered topically to postmenopausal women. Mean values are shown, and the SEs are indicated by error bars. *p<0.05 was considered significantly different from baseline values (Student's t-test).
The nanoformulation of progesterone presented a high negative average zeta potential of -54.2 mV (Figure4A). The zeta potential is an important factor for evaluating the stability of a nanoemulsion. It is a function of the particle surface charge, which modulates the magnitude of the electrostatic repulsion between particles. In general, particles are considered stably dispersed when the zeta potential is below -30 mV or above 30 mV due to the electric repulsion between the nanoparticles.
Hormone particle size measurementsThe mean particle sizes of the progesterone nanoparticles were measured by DLS (Figure4B). The progesterone nanoparticles showed a homogenous size distribution with a mean diameter of 150-450 nm.
The physical stability of the progesterone nanoparticles was also evaluated by examining changes in mean particle sizes during storage for 2 months at room temperature. The progesterone nanoparticles did not show statistically significant changes in their mean diameter (p>0.05) when stored at room temperature for 2 months. This long-term stability study indicates good physical stability, suggesting that the progesterone particles are stable during long-term storage.
Progesterone nanoparticle morphology as assessed by SEMSEM is a simple and practical method by which to evaluate the mean size and surface morphology of particles. The morphology of the progesterone nanoparticles was determined by SEM (Figure4C). The progesterone particles were almost spherical and uniform in shape with smooth surfaces. The mean diameter was 100-600 nm, and there was no visible aggregation of particles (Figure4C).
Depth and concentration of progesterone particles in skin layersRaman confocal spectroscopy was used to analyze the depth and concentration of progesterone nanoparticles in skin layers after transdermal application. The skin depth and concentration of progesterone was measured in the stratum cornum (SC), viable epidermis (EPI), and dermis (D) at 1, 3, 6, 21, and 24 hours (Figure4D).
DISCUSSIONConfocal Raman spectroscopy analysis was able to show a stable permeation of the hormones through the skin layers during the 24 hours period. This transdermal protocol was the basis for the development of an innovative proposal (Figures1 and 4D) using the nanostructured formulation used previously by our research group (3,4). This study represents the first use of confocal Raman spectroscopy to validate the transdermal absorption of estrogens and progesterone administered through the skin in postmenopausal women. This methodology explains the effect of the Biolipid-B2® enhancer used to continuously deliver estrogen and progesterone nanoparticles over a 24-hour period. Through this technique, it was possible to measure in vivo the depth and concentration of hormone nanoparticles (Figure1).
Transdermal HRT did not alter systolic and diastolic blood pressure in the treated subjects (Table1). During the study, no antihypertensive medications were prescribed. These results are consistent with other studies showing that transdermal HRT did not have any impact on blood pressure (4,9).
This paper shows for the first time the changes in circulating estradiol and FSH after 60 months of transdermal HRT. In addition, no vaginal bleeding was reported and there was no patients with increased endometrial thickness.
Previous studies reported that HRT increases the risk of breast cancer (11-12). However, none of the 66 postmenopausal women treated with this protocol had a BI-RADS classification of 4 or 5 after 60 months of treatment. Another important outcome that was assessed was weight gain, which was not observed in the 60-month follow-up period (Table1).
First-pass metabolism is often associated with poor bioavailability and other adverse effects (1-4). Recent studies have shown that the transdermal route is the best way to treat menopausal symptoms (3). When HRT is administered by other routes in postmenopausal women, the incidence of heart disease increases (10).
The cardiovascular risks associated with hormone replacement therapies (HRTs) differ according to the route used. There is strong evidence that transdermal estradiol has a cardioprotective effect. This effect may be due to its lack of effect on the coagulation cascade and resistance to activated protein C, which reduces the number of cardiovascular events compared with that in non-users (11).
In the present study, we demonstrated the safety and efficacy of a transdermal nanoparticulated hormone used as a stable and controlled release system to treat menopausal symptoms. One limitation of our study is that we were not able to evaluate additional advantages of this therapy.
A previous study showed that transdermal estrogen has a beneficial effect on blood pressure. We showed that the transdermal HRT protocol did not negatively impact blood pressure. These outcomes are consistent with previous studies that used the transdermal route for administration of HRT (3,13).
The protocol used in this study was an effective strategy for relieving postmenopausal symptoms, especially for women in whom the adverse effects of orally administered drugs are an inhibitory factor. We demonstrated that 60 months of treatment with this transdermal formulation significantly reduced postmenopausal symptoms compared with the baseline symptoms (Table1). The findings also suggested the efficacy of this protocol in restoring FSH levels (Figure3).
The transdermal hormone enhancer Biolipid-B2® is a vehicle used for delivering nanoparticle hormones into the 3 skin layers (3,4). In Brazil, this enhancer has been used in more than 20 states and has been approved by the Brazilian Drug Regulatory Agency (ANVISA).
The zeta potential value of -54.2 mV (Figure4A) suggests that the hormone nanoparticles are physically stable. Their stability is due to the electrostatic repulsion conferred by the chemical nature of the lipid matrix and possibly the adsorption of negatively charged ions onto the nanoparticle surfaces.
This trial demonstrated that nanoemulsion hormone treatment effectively reduced symptoms and significantly restored the serum levels of estradiol and FSH. These outcomes support the continued investigation of transdermal nanoparticulated hormones as a potential therapeutic agent in postmenopausal therapy.
ACKNOWLEDGMENTSWe gratefully acknowledge the financial support of Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq Brazilian Agency for Scientific and Technological Development (Post-Doctoral Scholarship Proc #151080/2010-0 and 202316/2011-4 and Research Scholarship #310483/2012-3). We also thank Rector Samela Gomes and Jorge Bachur for providing the graphical design.
AUTHOR CONTRIBUTIONSBarros B, Umbelino S, Freitas A, and Guerreiro S collected the data. Ruela R and Borges BC were responsible for the bibliographic review and data analysis. Lyra A and Botelho MA performed the statistical analysis. Queiroz DC and Fechine P were responsible for acquiring the SEM images and performing the nanosizer analysis. Almeida JG, Quintans Jr. L, and Botelho MA conceived the project and wrote the manuscript. Freitas A critically revised the content of the manuscript.
No potential conflict of interest was reported.