Glucagonoma syndrome is a rare disease that is usually associated with an underlying neuroendocrine tumor. Necrolytic Migratory Erythema (NME) has been associated with intestinal malabsorption disorders, hepatic cirrhosis, chronic pancreatitis, inflammatory bowel disease, and non-pancreatic malignancies, but may not always be associated with glucagonoma. In 1979, Mallinson and co-workers coined the term glucagonoma syndrome to describe alpha-cell pancreatic tumors associated with a characteristic erosive skin eruption, termed Necrolytic Migratory Erythema by Wilkinson. NME is characterized by an irregular annular eruption with serpiginous advancing borders, erosion, and crusting, resulting in a scalded appearance. The eruption has a cyclical nature with concurrent lesions at different levels of healing.
Here, we report two patients with NME associated with hyperglucagonemia and neuroendocrine tumor.
CASE 1This case involved a 66-year-old Brazilian male with an eight year history of recurrent cutaneous lesions.
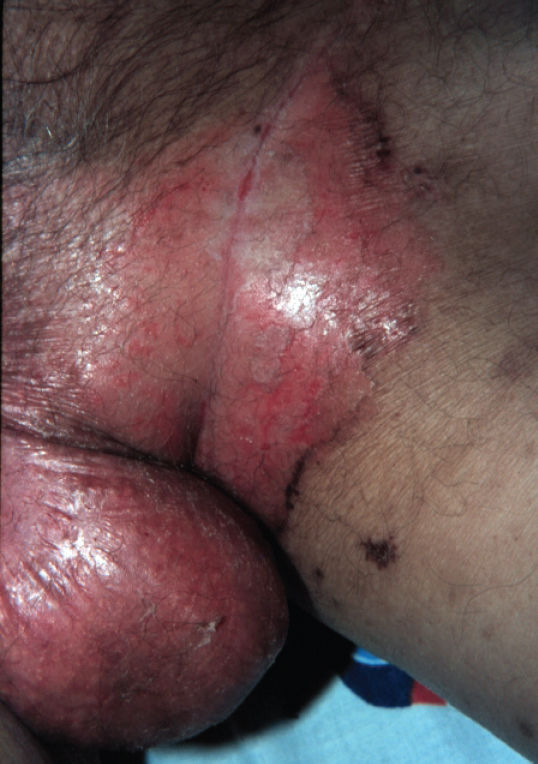
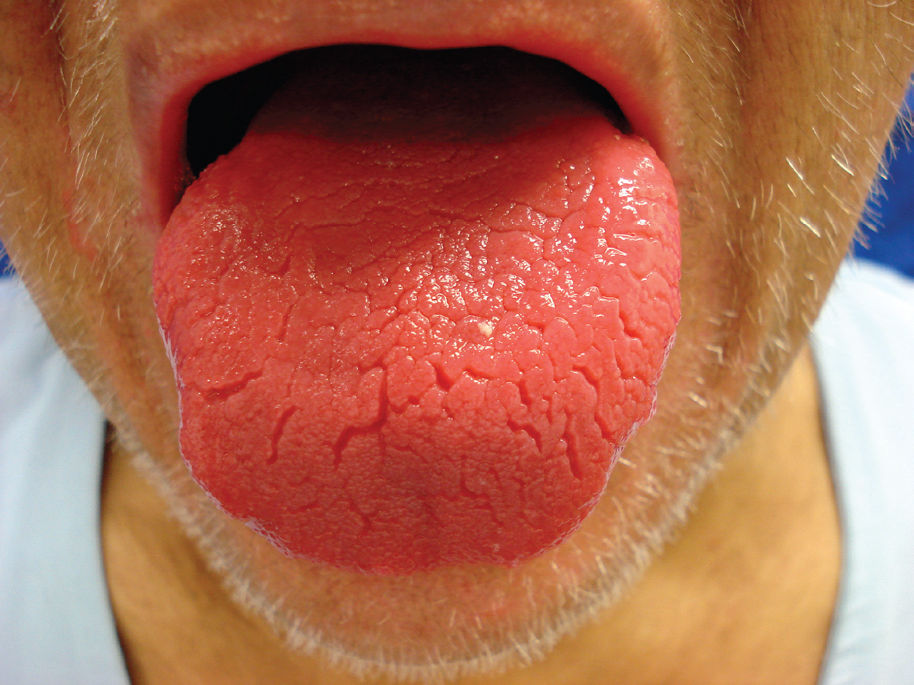
Abdominal surgery was performed due to colon cancer eight years before the dermatologic complaint. He also mentioned a significant alcohol history. Physical examination revealed erythematous, erosive scaling and crusted patches on the genital and groin area (Figure 1). He also presented with similar lesions in the perioral and periocular areas associated with angular cheilitis and a depapilated, bright red tongue (Figure 2). The patient denied intestinal symptoms and weight loss.
Laboratory testing showed normocytic normochromic anemia and slightly elevated levels of amylase and lipase. The patient had normal levels of serum zinc, folic acid, vitamin B12, albumin, globulins, alanine and aspartate tranferase. Hepatitis B and C and HIV serologies were negative. An elevated level of blood glucose (125mg/ml) was observed. The patient’s plasma level of glucagon was greater than 1,280 pg/ml (normal range: <60 pg/ml).
An abdominal computed tomography (CT) scan revealed a hypervascularized tumor measuring 6.1 x 3.8 cm in the body and the caudal portion of the pancreas, and an absence of liver metastasis. The patient was then subjected to a pancreatectomy, and the cutaneous lesions vanished one week after surgery.
CASE 2A 62-year-old Brazilian man was referred to our Dermatology Department complaining of two years of eroded skin. The eruption had a cyclical pattern, with periods of resolution.
During a weight loss investigation six months prior, a CT scan was performed and a pancreatic tumor with hepatic metastasis was confirmed. Surgical removal of the tumor was not possible. The patient had an episode of deep vein thrombosis associated with pulmonary embolism two months prior to being referred to our department earlier, and was taking marevan.
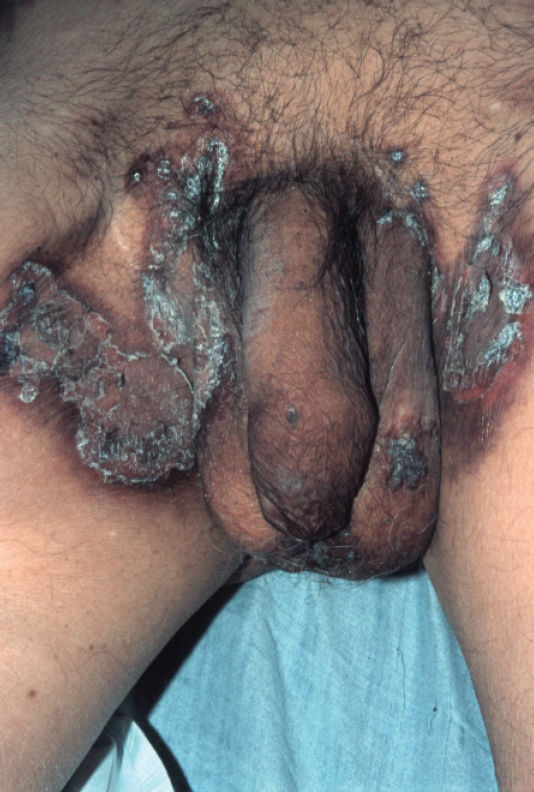
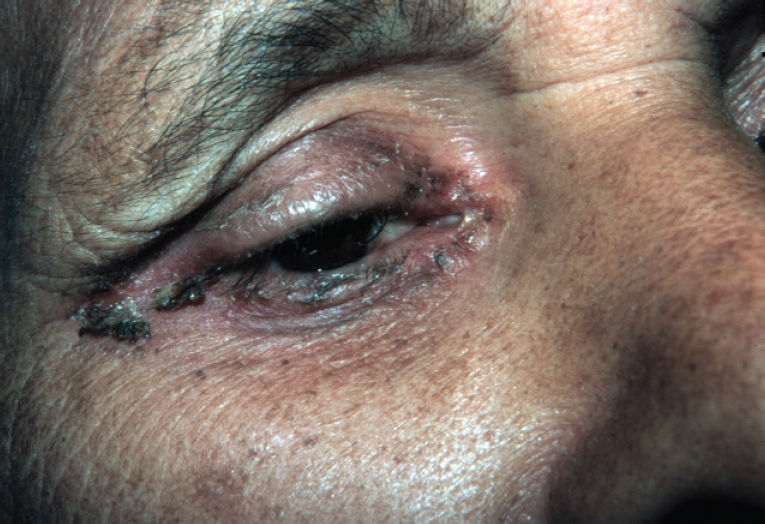
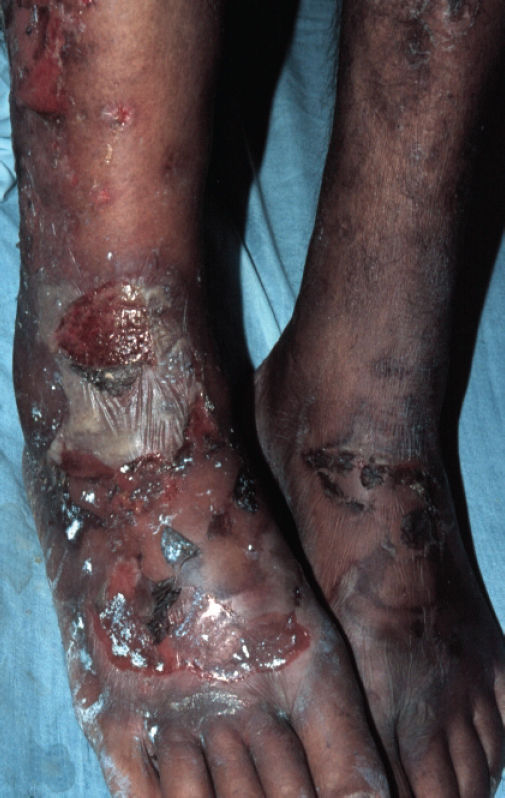
Physical examination revealed erythematous scaling and crusted plaques, with exulceration on the edges of the lesions, involving the groin and genital areas (Figure 3) and the periorbital area (Figure 4). He also presented with tense bullous lesions with purulent content and pellagroid eczematous plaques on the anterior surface of the legs and feet, associated with purpura and edema (Figure 5). Angular cheilitis and a depapilated red tongue were also present. In addition to the skin changes, the patient complained of weight loss and watery diarrhea.
Laboratory testing revealed normocytic normochromic anemia, hypoalbuminemia and elevated levels of amylase, lipase, alkaline phosphatase, gamma glutamyl transferase, alanine and aspartate transferase. Plasma levels of glucose and zinc were normal. The patient’s fasting plasma glucagon level was 1,280 pg/ml (normal range: <60 pg/ml), and amino acid levels were decreased.
An ultrasound-guided needle biopsy of a hepatic lesion was performed, and histopathological examination and immunohistochemistry revealed a neuroendocrine tumor.
Histopathological examination of a purpuric skin lesion showed extravasated erythrocytes on the superficial dermis and hyaline thrombus on the vascular lumen of on the deep dermis. These findings were attributed to the medication taken by the patient.
Palliative treatment with interferon alpha and octreotide LAR was proposed, but the patient presented an erythematous rash that was attributed to interferon alpha. Therefore, the offending drug was suspended. Octreotide LAR, 20 mg/month, was continued with complete resolution of the skin lesions. The patient was discharged and prescribed ambulatory chemotherapy.
DISCUSSIONGlucagonoma arises from alpha cells of pancreatic islets of Langerhans and may appear as either a benign, localized alpha cell adenoma, or a slow growing metastasizing malignant tumor. Glucagonoma is associated with striking systemic clinical manifestations, referred to as the “Glucagonoma Syndrome.” Systemic manifestations of the syndrome are numerous, and include diabetes mellitus, anemia, venous thrombosis, skin rash (NME), weight loss, glossitis, cheilitis, diarrhea, steatorrhea, and psychiatric disorders.
The cause of skin changes in NME is unclear. Glucagon per se has not been found to be the direct cause because there are patients who present with NME without neuroendocrine tumors or hyperglucagonemia. However, normalization of glucagon concentration by surgery or somatostatin analogs almost invariably results in a rapid resolution of the skin lesions. Furthermore, there is a recent report of a case of iatrogenic NME after intravenous administration of glucagon for the treatment of persistent hypoglycemia13 due to an insulinoma. This case supports the hypothesis of glucagon leading to the skin lesions. Zinc, essential fatty acid, and amino acid deficiencies are all considered to be possible causes of NME. However, not all patients with skin lesions present with these metabolic changes, and not all patients with these metabolic changes present with resolution of the skin lesions after zinc, essential fatty acid, or amino acid supplementation.14
NME is a rare dermatosis that is usually associated with an underlying pancreatic islet cell tumor. The skin eruption may be the first manifestation of the disease, and its recognition may lead to a diagnosis, as in case 1. The eruption has a cyclic nature, with periods of skin lesion resolution, as observed in both of our cases. The lesions consist of erythematous scaling and crusting patches most frequently observed in areas of trauma, such as the groin, intergluteal, and genital areas. Bullous lesions may occur. Cheilitis and glossitis are very common mucosal manifestations. It is commonly observed that these patients present with a history of antibiotic or antifungal treatments without improvements of their skin conditions before the correct diagnosis is made.
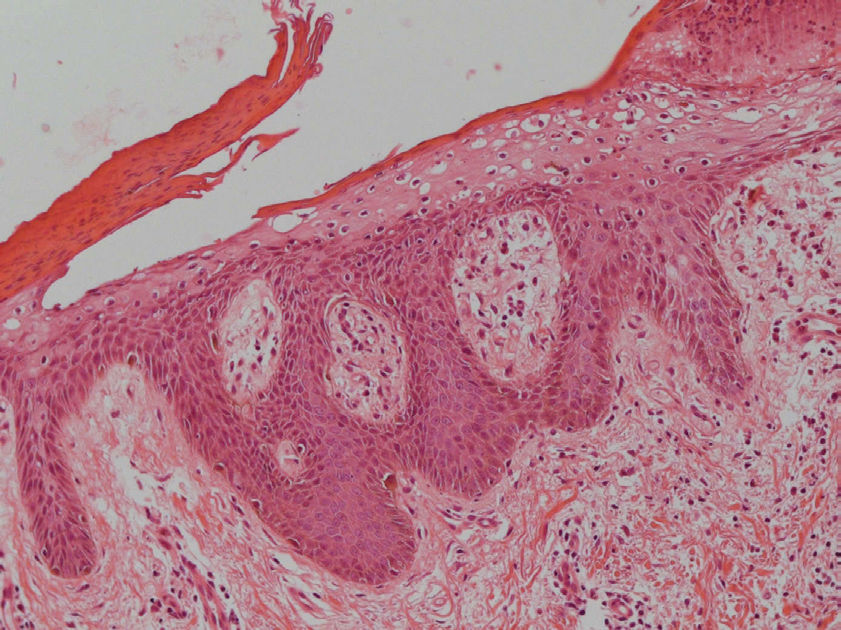
The histopathologic features of NME are nonspecific and may be seen in pellagra, necrolytic acral erythema, or zinc deficiency. Vacuolated, pale, swollen epidermal cells and necrosis of the superficial epidermis are characteristic (Figure 6). Biopsy specimens from the edges of active lesions are most likely to show the characteristic upper epidermal necrosis; however, many biopsy specimens do not have features that are typical, or even suggestive, of NME. Therefore, multiple biopsies are recommended when this diagnosis is suspected.
Diagnostic criteria of glucagonoma syndrome have been proposed by Stacpole and include demonstration of a tumor producing increased levels of glucagon, as revealed by special staining and increased circulatory levels of glucagon. In addition, the patient must meet at least one of the following criteria: (1) skin eruption, (2) diabetes mellitus, and (3) hypoaminoacidemia.
Glucagonomas show significant evidence of hypervascularity, so selective celiac and superior mesenteric arteriographies are the most reliable ways to detect the primary neoplasm if the abdominal CT scan does not disclose the tumor. Histopatologic confirmation of the glucagonoma occurs via immunohistochemistry, electronic microscope analysis, and in situ hybridization of glucagon messenger RNA. The absence of immunoreactivity for glucagon or glucagon messenger RNA transcripts in a metastatic focus may be due to tumor heterogeneity since glucagon may not be expressed in all metastatic foci. This could explain the negative immunohistochemistry for glucagon in case 2. Usually, the antigens used to assess neuroendocrine differentiation of tumors are synaptophysin and cromogranin. Hormones such as insulin, glucagon, vasointestinal polypeptide, somatostatin, and pancreatic polypeptide are used to determine the predominant hormone produced by tumors. In some cases, tumors produce more than one hormone.
The prognosis of the disease varies greatly according to the stage in which the tumor is diagnosed. By the time of diagnosis, 50–100% of patients already present with metastatic disease, and a cure is often impossible, as in case 2. The tumor is resistant to chemotherapy, and metastatic disease is often not amenable to surgical resection9. However, since this islet cell tumor is slow-growing, prolonged survival (more than 20 years) is possible, and in metastatic disease, most causes of death appear to be unrelated to the tumor.17
Successful palliative treatment is possible with long-acting somatostatin analogs,22,26 and/or interferon alpha. Supplementation with zinc, amino acids, and essential fatty acids appears to be beneficial in some cases.12,14
The patient in case 1 was a diagnostic challenge. Only skin lesions and mucosal changes were present at the time of diagnosis. In this case, the dermatologist was responsible for the pancreatic tumor diagnosis in the curable stage of disease.
In case 2, it was not possible to perform a surgical resection of the tumor, so only an improvement in the skin lesions was feasible.
We describe two cases that illustrated good outcomes of skin lesions in two different situations: case 1 was a patient with an early diagnosis and a curable disease and case 2 was a patient with a metastatic tumor. Both patients presented with resolution of skin lesions after beginning treatment.