Epidemiological studies indicate that approximately 5% of people previously classified as type 2 diabetes mellitus carriers and 10% of those considered as type 1 are, in fact, carriers of maturity-onset diabetes of the young (MODY), a subtype of non-insulin-dependent diabetes mellitus.1 This monogenic form of diabetes is characterized by early onset (usually under 25 years old), high penetrance and the autosomal dominant mode of inheritance.2,3
Six MODY genes have already been identified. The MODY 3 subtype, caused by mutations in the HNF-1α (hepatocyte nuclear factor-1 alpha) gene,4 is the most common Mendelian form of diabetes and it is found in more than 60% of the cases classified as MODY, accounting for 1–2% of all diabetic individuals.5 The diabetic phenotype of the carriers of this mutation is due to altered gene expression of pancreatic β-cells, mainly of the genes encoding insulin, the glucose transporter Glut2, amino acid transporters and some mitochondrial enzymes.6,7
The identification of the MODY genes has important clinical implications for patients, and their correct diagnosis is essential for a more appropriate treatment of the disease. The aim of this study was to examine a family suspected of carrying a type of MODY diabetes, searching for mutations present in this syndrome and its correlation with the clinical aspects of the patients.
This study received approval from the Ethics Committee of the Universidade Estadual de Ponta Grossa (COEP authorization no. 14/2009, protocol no. 00884/09) and signed consent was obtained from all patients. Three generations of a family from South Brazil with a history of progressive and early diabetes, characteristics of MODY 3,2,3,5,8 were analyzed.
EDTA anticoagulant venous blood samples were collected from the patients and the isolated genomic DNA9 was used as template in a polymerase chain reaction. Because MODY 3 is the most commonly occurring form of the disease in Brazil,10 oligonucleotides were synthesized for the flanking regions (introns) of the 10 exons of HNF-1α. The samples were subjected to electrophoresis and purified, and then submitted for automatic nucleotide sequencing.
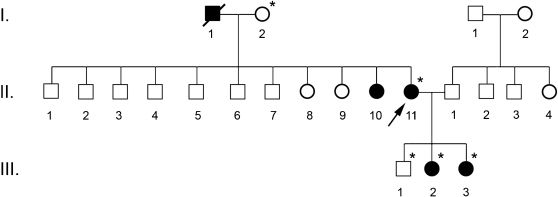
After nucleotide alignment, the presence of a mutation in exon 2 of the HNF1-α gene was detected in some members of the family: the proband and her daughters (Figure 1). The transition of a guanine to an adenine nucleotide at position 339 of codon 113 (TGG→TGA) has generated a nonsense mutation in this position (W113X), not yet described in the literature, which inhibits the formation of a functional protein in the affected individuals. More than 120 mutations have already been identified in the HNF1-α gene, e.g. missense, nonsense, frameshift and splice-site MODY mutations, in all exons analyzed.11 The majority of mutations in the HNF-1α protein lead to the formation of nonfunctional heterodimers with the product of the normal HNF-1α allele, preventing them from binding to DNA.
All the affected members analyzed in this study showed hyperglycemia at young ages, and the proband, now 38 years old, already manifests impaired vision and microcirculation, characteristics of a progressive syndrome, such as MODY 3. Some reports may explain the precocious manifestation of the disease in individuals of this study. According to Harries et al.12 the age of MODY 3 diagnosis is determined, in part, by the location of the mutation; mutations in the proximal segment of the HNF-1α gene should give rise to a more severe phenotype than mutations in the distal regions. Furthermore, the age at diagnosis of diabetes varies according to the type of the HNF-1α mutations: the median age at diagnosis was lower in patients with truncating mutations than in those with missense mutations.13
Functionally, the HNF-1α protein can be divided in three regions: amino-terminal dimerization domain (amino acids 1–32), homeodomain binding domain (203–276) and transactivation carboxyl-terminal domain (281–631).14 The mutations in this protein are more frequent in the homeodomain regions and in a near amino-terminal region, between residues 91 and 185,15,16 in agreement with our findings (mutation at amino acid 113). This region is responsible for binding and specificity in the interaction with DNA.15,17 Studies indicated that suppression of HNF-1α function affects β-cell metabolism leading to MODY 318 and concluded that normal function of the HNF-1α protein is required for correct transcription of the insulin gene.19
In conclusion, the data presented in this study describe a new mutation for MODY type 3 and suggest functional significance of this genetic alteration in the insulin secretion process, besides highlighting the diagnostic importance of this type of diabetes aiming the control and clinical follow up.
This study was financed by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Fundação Araucária (Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná) and, SETI/UGF (Secretaria de Estado da Ciência, Tecnologia e Ensino Superior/Unidade Gestora do Fundo do Paraná).