To determine the nature of hyaline membranes in different manifestations of diffuse alveolar damage, [pulmonary and extrapulmonary acute respiratory distress syndrome], and idiopathic [acute interstitial pneumonia].
MATERIALS AND METHODS:Pulmonary specimens were obtained from 17 patients with acute respiratory distress syndrome and 9 patients with acute interstitial pneumonia. They were separated into 3 different groups: (a) pulmonary diffuse alveolar damage (pDAD) (n = 8), consisting only of pneumonia cases; (b) extrapulmonary diffuse alveolar damage (expDAI) (n = 9), consisting of sepsis and septic shock cases; and (c) idiopathic diffuse alveolar damage (iDAD) (n = 9), consisting of idiopathic cases (acute interstitial pneumonia). Hyaline membranes, the hallmark of the diffuse alveolar damage histological pattern, were examined using various kinds of antibodies. The antibodies used were against surfactant apoprotein-A (SP-A), cytokeratin 7 (CK7), cytokeratin 8 (CK8), alpha smooth muscle actin (α-SMA), cytokeratin AE1/AE3 (AE1/AE3), and factor VIII-related antigen (factor VIII).
RESULTS:Pulmonary diffuse alveolar damage showed the largest quantity of hyaline membranes (12.65% ± 3.24%), while extrapulmonary diffuse alveolar damage (9.52% ± 3.64%) and idiopathic diffuse alveolar damage (7.34% ± 2.11%) showed intermediate and lower amounts, respectively, with the difference being statistically significant between pulmonary and idiopathic diffuse alveolar damage (P < 0.05). No significant difference was found for hyaline membranes Sp-A immunostaining among pulmonary (15.36% ± 3.12%), extrapulmonary (16.12% ± 4.58%), and idiopathic (13.74 ± 4.20%) diffuse alveolar damage groups. Regarding factor VIII, we found that idiopathic diffuse alveolar damage presented larger amounts of immunostained hyaline membranes (14.12% ± 6.25%) than extrapulmonary diffuse alveolar damage (3.93% ± 2.86%), with this difference being statistically significant (P < 0.001). Equally significant was the difference for progressive decrease of cytokeratin AE1/AE3 immunostaining in hyaline membranes present in the extrapulmonary diffuse alveolar damage (5.42% ± 2.80%) and idiopathic diffuse alveolar damage (0.47% ± 0.81%) groups (P < 0.001). None of the groups stained for cytokeratin CK-7, CK-8, vimentin, or α anti-smooth muscle actin.
CONCLUSIONS:This study showed that only the epithelial/endothelial components (SP-A, factor VIII, and AE1/AE3) of the alveolar/capillary barrier are present in hyaline membranes formation in the 3 groups of patients with diffuse alveolar damage. The significant difference in the expression of factor VIII-related antigen and cytokeratin AE1/AE3 in the expDA versus iDAD groups as well as the significant difference in the amount of hyaline membranes present in the pDAD versus iDAD groups are suggestive of a local and specific lesion with different pathways (direct, indirect, or idiopathic), depending on the type of diffuse alveolar damage.
Determinar a natureza da membrana hialina nas diferentes manifestações do dano alveolar difuso [pulmonar e extrapulmonar síndrome do desconforto respiratório] e idiopático [pneumonia intersticial aguda].
MATERIAIS E MÉTODOS:Espécimes pulmonares foram obtidos de 17 pacientes com SDRA e 9 pacientes com pneumonia intersticial aguda e separados em três diferentes grupos: (a) dano alveolar difuso pulmonar (DADp) (n=8) constituído por casos de pneumonia, (b) dano alveolar difuso extrapulmonar (DADexp) (n=9) constituído por casos de sepse e choque séptico e (c) dano alveolar difuso idiopático (DADi) (n=9) constituído por casos idopáticos (ou pneumonia intersticial aguda). As características das membranas hialinas do padrão histológico de dano alveolar difuso foram examinadas usando vários tipos de anticorpos. Os anticorpos usados foram surfactante apoproteina A (SP-A), anti-citokeratina 7 (CK7), citokeratina 8 (CK8), alfa actina de músculo liso (a-SMA), citokeratina AE1/AE3 (AE1/AE3) e antígeno relacionado ao fator VIII (Fator VIII).
RESULTADOS:Observaram-se aumentos maiores da quantidade de membrana hialina no dano alveolar difuso pulmonar (12.65 ± 3.24%), intermediários no dano alveolar difuso extrapulmonar (9.52 ± 3.64%) e baixos no dano alveolar difuso idiopático (7.34 ± 2.11%) respectivamente, esta diferencia foi estatística significante entre o dano alveolar difuso pulmonar e o dano alveolar difuso idiopático (p<0.05). Não se encontrou significância estatística para a quantidade de imunomarcação de Sp-A nos grupos de dano alveolar difuso pulmonar (15.36 ± 3.12%), extrapulmonar (16.12 ± 4.58%) e idiopático (13.74 ± 4.20%). Com relação ao Fator VIII, nós encontramos maiores aumentos da imunomarcação da membrana hialina no grupo dano alveolar difuso idiopático (14.12 ± 6.25%) do que no dano alveolar difuso extrapulmonar (3.93 ± 2.86%), com significância estatística (p<0.001). Da mesma forma houve um aumento progressivo da imunomarcação da membrana hialina com citokeratina AE1/AE3 nos grupos de dano alveolar difuso extrapulmonar (5.42 ± 2.80%) e dano alveolar difuso idiopático (0.47 ± 0.81%) (p<0.001). Nenhuns dos grupos marcou para as citokeratina CK-7, CK-8 ou para a vimentina ou actina de músculo liso.
CONCLUSÕES:Este estudo só mostra os componentes epitelio/endotelio (SP-A, Fator VIII e AE1/AE) da barreira alvéolo/capilar que estão presentes na formação da membrana hialina nos três diferentes grupos, sugerindo que as diferenças na sua patogênese dependem da via do ferimento (direto, indireto, ou idiopático); que dependem da causa do dano alveolar difuso. As diferenças na expressão do antígeno relacionado ao Fator VIII e nas citoqueratinas AE1/AE3 no grupo DADexp versus DADi, bem como as diferenças entre HM presente no grupo DADp versus DADi sugerem a ocorrência de lesões específicas com vias diferentes (direta, indireta ou idiopática) dependentes do tipo de DAD.
The pathogenesis of acute respiratory distress syndrome (ARDS) leading to increased alveolar capillary permeability and diffuse inflammatory infiltration, manifested clinically as refractory hypoxemia in association with decreased lung compliance and radiographically as bilateral pulmonary infiltrates, is not well understood.
Two pathways are supposed to be involved in ARDS pathogenesis: (a) the pulmonary or direct effects of an inhalatory insult on lung cells and (b) the extrapulmonary or indirect result of an acute systemic inflammatory response; however, the causes of ARDS still pose a challenge.
In this regard, many have studied the differences between pulmonary ARDS (pARDS) and extrapulmonary ARDS (expARDS) in a quest for what might be related to the dramatic mechanisms of hypoxemia. In 1998, Gattinoni et al1 suggested that patients suffering direct pulmonary insults show lower compliance and have alveolar units that are less susceptible to recruitment by mechanical ventilation than do those with extrapulmonary initiating processes.
In 1999, Pelosi et al2 analyzed chest radiographs by using appropriate scores and found that patients with direct lung injury presented increased patchy densities and edema scores when compared to patients with indirect lung injury, whereas the amount of hazy and extensive densities were similar in both groups. Goodman et al3 analyzed CT scans and found that pARDS tends to be asymmetric, with a mix of consolidation and ground-glass opacification, whereas expARDS has a predominantly symmetric ground-glass opacification. Similar findings have been reported in which less recruitable lung regions in patients with pneumonia are described.4,5
Because the lung injury in ARDS results in diffuse alveolar damage (DAD), a group of morphologic parameters have been targeted as potentially useful by Hoelz et al;6 among these, hyaline membranes have been investigated. Hyaline membrane (HM) was first described by Hamman and Rich in acute interstitial fibrosis7 and since then it has been seen as an acute histological parameter occurring in a progressive, fatal, and distinct form of interstitial pneumonia8,9 classified by the American Thoracic Society/European Respiratory Society (ATS/ERS)10 as acute interstitial pneumonia.
Immunostaining of HM is heterogeneous, suggesting that it may be formed through different mechanisms in various types of diffuse interstitial pneumonia.11 In order to validate the importance of HM and explore the quantitative relationship between this factor and parenchymal changes in diffuse alveolar damage, as well as to characterize the immunostaining features of HM, we studied HM in lungs of patients with different manifestations of DAD [pulmonary and extrapulmonary (ARDS) and idiopathic acute interstitial pneumonia (AIP)].
MATERIALS AND METHODSPatients and Lung TissuePulmonary specimens were obtained from 26 patients with DAD by surgical lung biopsy and necropsy from 1998 to 2003. All of the patients exhibited clinical, radiological, and physiological alterations consistent with ARDS according to the 1992 American-European Consensus12 and had received the definitive pathological diagnosis of diffuse alveolar damage (DAD). Patients with ARDS were separated into 3 groups: (a) pulmonary DAD (pDAD) (n = 8), consisting only of pneumonias cases; (b) extrapulmonary DAD (expDAD) (n = 9), consisting of sepsis and septic shock cases; and (c) idiopathic DAD (iDAD) (n = 9), consisting of idiopathic cases (or acute interstitial pneumonia), according to the criteria outlined in the American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias.10 Pulmonary tissues were fixed in 10% phosphate-buffered formalin solution, cut into slices, embedded in paraffin, and sectioned at a thickness of 4 µm for histological evaluation with hematoxylin and eosin (H&E). From the H&E sections of each case, representative sections containing HM were chosen for immunohistochemical analysis.
Hyaline membrane evaluationHyaline membranes were evaluated by immunohistochemical staining using the avidin-biotin immunoperoxidase complex technique. The antibodies used were antisurfactant apoprotein-A (SP-A) (Clone PE10; Dako; 1:800 dilution), which is used to differentiate type II pneumocytes and Clara cells; anticytokeratin 7 (CK7) (Clone OV-TL 12/30; Dako; 1:100 dilution), used to differentiate type I and II pneumocytes and bronchial epithelial cells; anticytokeratin 8 (CK8) (Dako; 1:200 dilution), used to differentiate simple epithelia; antivimentin (Dako; 1:800 dilution), used to differentiate mesenchymal cells; anti-alpha smooth muscle actin (α-SMA) (Novocastra; 1:500 dilution), anticytokeratin AE1/AE3 (AE1/AE3) (Dako; 1:100 dilution), used to differentiate keratinized epidermis, simple epithelia, and squamous stratified epithelia of internal organs; and antifactor VIII-related antigen (anti-Factor VIII) (Dako; 1:400 dilution), used to differentiate endothelial cells. Positive and negative controls were stained in parallel, and staining processes were performed according to the manufacturer’s instructions.
To quantify the immunostaining of HM, we counted 500 lines (or 10 fields) per case at X400 magnification in each case, using a conventional stereological method. Briefly, this method is a line-counting procedure that consists of a reticulated eyepiece made of 100 points and 50 lines, each one with a length of 25 mm at a magnification of X400, coupled to a conventional light microscope. Counting was performed by using a cascade progressive sampling approach. The error in this procedure is estimated as being lower than 5%.13 The numbers of positively and negatively stained HM were averaged to obtain a final result, expressed as a percentage.
We considered negative cases of HM immunostaining to be those with a lower staining level than controls or cases with a staining level resulting in less than 10 lines overlying HM.
Statistical analysisFor the quantification of HM, we used the 1-way ANOVA procedure for analysis of variance of immunohistochemical stains and their distributions, as well as for analyzing the tissue and air density in the 3 groups (pDAD, expDAD, and iDAD) Differences among the means were compared a priori by Levene’s test for homogeneity of variance and then by the Dunnett T3 multiple comparisons post hoc tests. The level of significance was set at 0.05. The data were analyzed using the SPSS for Windows program, release 11.0.13
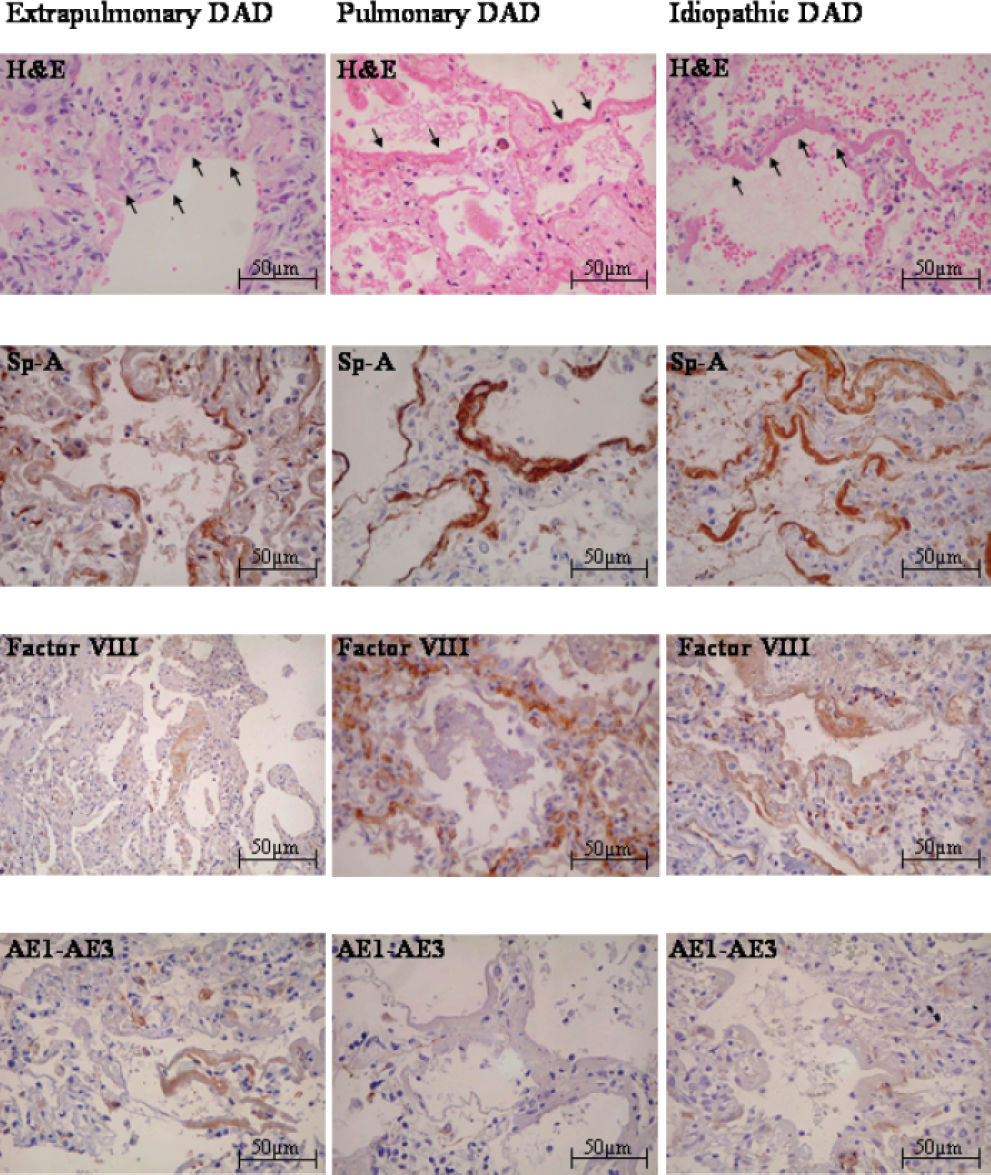
RESULTSHistopathological evaluationFigure 1 shows the histological features of HM in pDAD, expDAD, and iDAD.
Hyaline membrane (HM) (arrows) in 3 groups of diffuse alveolar damage (DAD)—extrapulmonary DAD, pulmonary DAD, and idiopathic DAD—is eosinophilic material lining alveolar surfaces that is presented in different ways depending on the group studied. Hyaline membrane appeared as a thick layer near basal membrane (in all groups), as a variable thin layer on the alveolar surface (in extrapulmonary DAD), and as interstitial fragments in cases in which there was less alveolar space (especially in idiopathic DAD and pulmonary DAD). Hyaline membrane of all 3 groups reacted with antibodies to surfactant apoprotein-A (SP-A). The extrapulmonary DAD group reacted with anti-Factor VIII in a few cases and was the only group that stained for AE1/AE3. The pulmonary DAD group had an intermediate stain level for factor VIII, but did not react with anti-AE1/AE3. All the HM of the idiopathic DAD group stained for Factor VIII and also did not react with anti-AE1/AE3.
Hyaline membrane was the major histopathological alteration in all 26 cases studied. It was observed as an eosinophilic material lining alveolar surfaces, presenting in different ways depending on the group studied. For instance, HM appeared as a continuous thin layer on the alveolar surface in expDAD or discontinuous and fragmented in iDAD and pDAD. Type I and II pneumocytes (PI and PII respectively), macrophages, blood cells, and edema, bordering or mixed with HM, were also present.
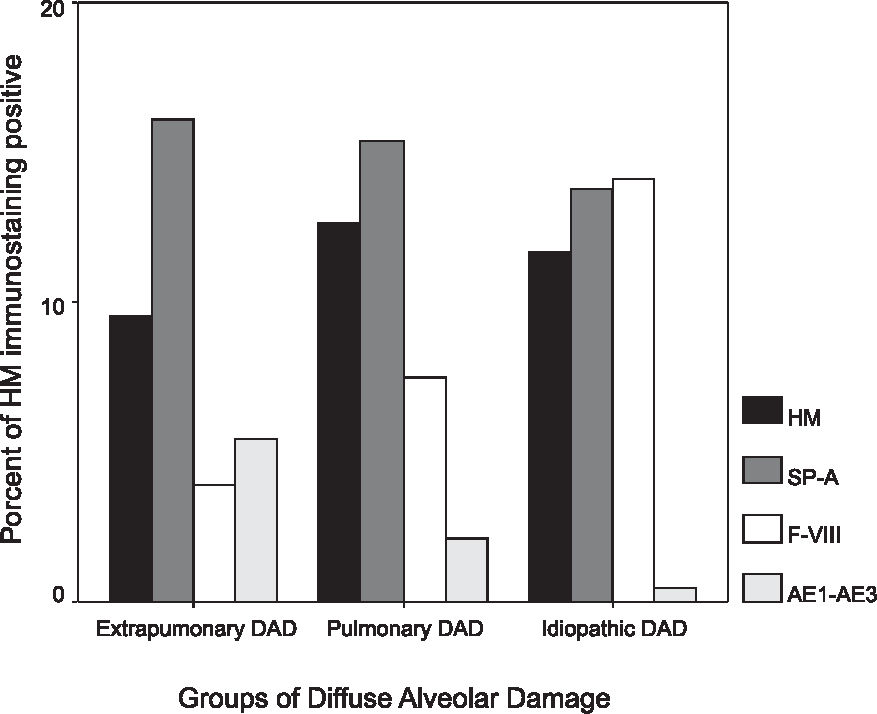
As shown in Figure 2 and Table 1, pDAD showed the largest quantity of HM (12.65% ± 3.24%), while epDAD (9.52% ± 3.64%) and iDAD (7.34% ± 2.11%) showed intermediate and lower amounts, respectively, with this difference being statistically significant between pulmonary and idiopathic DAD (P < 0.05).
Graphic illustration and quantitative analysis of hyaline membrane (HM) under hematoxylin & eosin staining and immunostained for surfactant apoprotein-A (SP-A), Factor VIII (F-VHI), and cytokeratin AE1/AE3 (AE1/AE3) in extrapulmonary DAD, pulmonary DAD, and idiopathic DAD. The 1-way ANOVA procedure was used for analysis of variance of immunohistochemical stains and their distributions, and for the quantification of HM
Results of the statistical analysis of the quantitation of hyaline membrane (HM) and the 3 antigens that showed immunohistochemical positivity among the 3 groups of patients with DAD (mean ± SD)
The results of the HM immunostaining quantitation are shown in Figure 2 and Table 1. No statistical differences were found for HM Sp-A immunostaining among pulmonary (15.36% ± 3.12%), extrapulmonary (16.12% ± 4.58%), and idiopathic (13.74% ± 4.20%) DAD groups. Regarding factor VIII, we found that iDAD presented larger amounts of immunostained HM (14.12% ± 6.25%) than expDAD (3.93 ± 2.86%), with this difference being statistically significant (P < 0.001). Equally significant was the difference for the progressive decrease of cytokeratin AE1/AE3 immunostaining in HM present in the expDAD (5.42% ± 2.80%) versus iDAD (0.47% ± 0.81%) groups (P < 0.001).
Immunostaining for cytokeratin CK-7, CK-8, vimentin, and a-SMA HM was negative in the pDAD, epDAD, and iDAD groups.
DISCUSSIONHyaline membrane is the histological hallmark of ARDS and AIP and results from acute inflammatory processes in interstitial and alveolar compartments.15 The composition of HM is not completely known, and its pathogenesis is still controversial.10 In this study, we investigated the immunohistochemical features of HM in 3 groups of patients who presented different manifestations of DAD [pulmonary ARDS, extra pulmonary ARDS, and idiopathic DAD (AIP)].
Our findings show that the surfactant apoprotein-A (SP-A), a marker of type II pneumocytes, is strongly positive in the HM of all groups. This staining also allowed a better identification of the HM pycnotic nuclei, which were probably derived from injured type II pneumocytes.
When factor VIII was studied through the staining of endothelial cytoplasmic proteins, we observed strongly positive staining in iDAD, which was also coincident with the intensity of staining for SP-A, showing that HM in this group results from the simultaneous destruction of type II pneumocytes and endothelium. Pulmonary DAD, a group with intermediate factor VIII expression, was found only in pneumonia cases. These cases are usually characterized by intense inflammatory process with cytokines (TNF-± and interleucin-1) and complement (C5-a and C5b-9) activation, resulting in overexpression of macrophages and neutrophils with substantial superoxide production and endothelial destruction.16,17 On the other hand, the lower amount of factor VIII expression in the expDAD group probably coincides with minor endothelium injury. In our study, this group was composed of only sepsis or septic shock cases, usually characterized by systemic (but not local) inflammatory injury, with vascular dilatation and less capillary exchange. This finding had been previously shown by Grandel and Grimminger,18 who demonstrated that endothelial alteration might occur by cell dysfunction (including type I and II pneumocytes) without vascular destruction.
Cytokeratin AE1/AE3 staining is positive in almost all epithelia because it reacts with keratinized epithelium, stratified desquamative epithelium of internal organs, proliferating keratinocytes, and simple epithelium. In our study, cytokeratin AE1/AE3 was highly positive in expDAD (sepsis or septic shock cases), indicating the occurrence of epithelial cells, whose integrity is necessary for adequate air exchange. In contrast, the lower quantity of cytokeratin AE1/AE3 found in pulmonary and idiopathic DAD suggests minimal injury to epithelial cells. In addition, our study showed that other epithelial markers, such as CK-7 and CK-8, were not expressed in the HM. Cytokeratin 7, an intermediated filament of glandular epithelium protein, is usually present in type I and II pneumocytes, whereas CK-8 is present in simple epithelium such as ductal carcinomas of the breast and is usually present at minimal amounts in the alveolar epithelium. These findings suggest that the alveolar epithelium may have a small role in HM pathogenesis, because among the 3 markers for epithelium used (CK-7, CK-8, and AE1/AE3), AE1/AE3 was the only one that showed positive immunostaining in the expDAD group.
The interstitial markers used in this study such as vimentin, specific for mesenchymal cells, and anti-α smooth muscle α actin, specific for this kind of muscle, were all negative for immunostaining, showing that HM usually does not contain mesenchymal components.
In conclusion, this study shows that only the epithelial/endothelial components (SP-A, factor VIII, and AE1/AE3) of the alveolar/capillary barrier are present in HM formation in the 3 groups of patients with DAD. The significant difference in the expression of factor VIII-related antigen and cytokeratin AE1/AE3 in the expDAD versus iDAD groups as well as the significant difference in the amount of HM present in the pDAD versus iDAD groups are suggestive of a local and specific lesion with different pathways (direct, indirect, or idiopathic), depending on the type of the DAD.
ACKNOWLEDGEMENTSWe are grateful to Sandra de Moraes Fernezlian for her careful and dedicated work in the immunohistochemical procedures.