Remote ischemic perconditioning is the newest technique used to lessen ischemia/reperfusion injury. However, its effect in hypertensive animals has not been investigated. This study aimed to examine the effect of remote ischemic perconditioning in spontaneously hypertensive rats and determine whether chronic treatment with Olmesartan could influence the effect of remote ischemic perconditioning.
METHODS:Sixty rats were randomly divided into six groups: vehicle-sham, vehicle-ischemia/reperfusion injury, vehicle-remote ischemic perconditioning, olmesartan-sham, olmesartan-ischemia/reperfusion and olmesartan-remote ischemic perconditioning. The left ventricular mass index, creatine kinase concentration, infarct size, arrhythmia scores, HIF–1α mRNA expression, miR-21 expression and miR-210 expression were measured.
RESULTS:Olmesartan significantly reduced the left ventricular mass index, decreased the creatine kinase concentration, limited the infarct size and reduced the arrhythmia score. The infarct size, creatine kinase concentration and arrhythmia score during reperfusion were similar for the vehicle-ischemia/reperfusion group and vehicle-remote ischemic perconditioning group. However, these values were significantly decreased in the olmesartan-remote ischemic perconditioning group compared to the olmesartan-ischemia/reperfusion injury group. HIF–1α, miR-21 and miR-210 expression were markedly down-regulated in the Olmesartan-sham group compared to the vehicle-sham group and significantly up-regulated in the olmesartan-remote ischemic perconditioning group compared to the olmesartan-ischemia/reperfusion injury group.
CONCLUSION:The results indicate that 1 the protective effect of remote ischemic perconditioning is lost in vehicle-treated rats and that chronic treatment with Olmesartan restores the protective effect of remote ischemic perconditioning; 2 chronic treatment with Olmesartan down-regulates HIF–1α, miR-21 and miR-210 expression and reduces hypertrophy, thereby limiting ischemia/reperfusion injury; and 3 recovery of the protective effect of remote ischemic perconditioning is related to the up-regulation of HIF–1α, miR-21 and miR-210 expression.
Ischemia/reperfusion (I/R) injury is a widespread phenomenon that occurs in all clinical scenarios when the blood supply to tissues is interrupted and then restored. The possibility of severe I/R injury should be considered during acute arterial occlusions, valve replacements and organ transplantations. Therefore, reducing the degree of I/R injury has great clinical significance. Cardioprotective strategies, such as ischemic preconditioning (IPC) and ischemic postconditioning (IPOST), effectively lessen I/R injury and reduce the myocardial infarct size. Recent studies have demonstrated that brief repetitive ischemia and reperfusion of a remote organ or limb during target organ ischemia, termed remote ischemic perconditioning (RIPER), could generate protection for the target organ challenged by lethal ischemia 1. Compared to other conditioning techniques used to lessen I/R injury (IPC and IPOST), RIPER is a noninvasive technique and does not prolong the operating time.
Hypoxia occurs following acute and chronic ischemia. The major effector of the hypoxic response in metazoan is hypoxia-inducible factor–1 (HIF–1), a heterodimeric complex containing an oxygen-labile α-subunit and a constitutively expressed β-subunit 2. An increase in HIF–1α is one of the first adaptive responses of human myocardium to myocardial ischemia or infarction 3. Moreover, HIF–1α plays a central role in IPC-mediated and IPOST-mediated cardioprotection 4,5.
MicroRNAs (miRs) are a class of endogenous 20–24 nucleotide non-protein-coding RNAs that regulate eukaryotic gene expression at the post-transcriptional level 6. In addition to the canonical HIF–1 regulation, a specific set of miRs, termed hypoxamirs, play important roles in hypoxic adaptation 7. During the past 5 years they have emerged as regulators of I/R injury as well as IPC and IPOST 8.
Hypertension, as a common cardiovascular risk factor, increases myocardial load/mechanical stress and may thereby exacerbate the outcome of an I/R insult to the heart 9. Numerous experimental studies have revealed that the cardioprotective effects of IPC and IPOST have been diminished or abrogated in the presence of hypertension 10,11. However, little is known about the effects of RIPER on infarct size in hypertensive animals.
Angiotensin II receptor blockers (ARBs) are a class of drugs with increasing use in treating hypertension. Olmesartan, as a new ARB, has shown beneficial effects beyond decreasing blood pressure (BP). Clinical studies have demonstrated that olmesartan could reverse the remodeling and angiosclerosis of small resistance vessels, reduce levels of inflammatory factors, inhibit the progression of atherosclerosis, and reduce the risk of major adverse cardiac and cerebrovascular events 12–14. Swindle found that compared to other ARBs (valsartan, losartan and irbesartan), olmesartan is associated with a lower risk of cardiac events and lower healthcare resource utilization and costs 15.
Considering that most hypertensive patients undergoing I/R injury are pretreated with antihypertension agents (such as olmesartan) and that these agents may potentially modify intracellular signaling relevant to cytoprotection, and thus myocardial responses to cardioprotective strategies, the objectives of the present study are (i) to examine the effect of a protocol of RIPER against infarct size in spontaneously hypertensive rats (SHRs); (ii) to determine whether chronic treatment with Olmesartan can influence the effects of RIPER in hypertensive rat hearts; and (iii) to examine whether HIF–1α, miR-21 and miR-210 are involved in the protective effects afforded by olmesartan and RIPER.
METHODSEthicsAll of the rats were handled in accordance with the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The experiment was approved by the Animal Care and Use Committee of Taishan Medical University and was carried out in the Key Laboratory of Atherosclerosis in Universities of Shandong (Taishan Medical University).
Animal housing and surgical preparationSHRs, aged 12 weeks, were used in this study. SHRs were provided by Vital River Laboratories and were housed in stainless steel cages, six to a cage, with food and water available ad libitum and natural illumination. Sixty SHRs were randomly divided into two groups: SHRs in the vehicle group (n=30) were given vehicle besides normal diet for four weeks, whereas those in the olmesartan group received olmesartan (5 mg/kg) by stomach rearing every day alongside a normal diet for four weeks. BP was assayed every week.
After an intraperitoneal injection of sodium pentobarbital (45 mg/kg), the body weight (BW) of SHRs was recorded. Then, each rat was fixed in the supine position on an operation platform with a heating pad (36.0−38.0°C) to maintain body temperature. The animal was tracheally intubated and mechanically ventilated using a small animal ventilator (Animal Respirator ALC-V8, Alcott Biotech, Shanghai, China). The chest was opened by a left thoracotomy through the fourth intercostal space. After removing the pericardium and exposing the heart, a 6–0 monofilament suture was placed around the proximal portion of the left anterior descending coronary artery (LAD) and passed through a short piece of tubing (PE50) to create a reversible snare. Following stabilization of the heart, coronary occlusion was initiated by clamping the snare onto the epicardial surface directly above the coronary artery. Successful occlusion of LAD was confirmed by the presence of ST segment elevation on the ECG and a change in ventricular color from fresh red to dark red. After 40 min of occlusion, reperfusion was achieved by loosening the snare and confirmed by a marked hyperemic response at reperfusion.
Experimental protocolTwo groups of animals were randomly assigned to one of three subgroups: (i) the sham subgroup (n=10), where SHRs underwent the same surgical procedures except that the suture passed under the LAD without being tightened; (ii) the I/R subgroup (n=10), where the LAD of SHRs was ligated for 40 min followed by 180 min of reperfusion and (iii) the RIPER subgroup (n=10), where during the occlusion of the LAD and before the reperfusion, the left hind limb of SHRs was subject to three cycles of 5 min of ischemia followed by 5 min of reperfusion.
Measurement of tail artery blood pressureSystolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in conscious SHRs every week by tail-cuff plethysmography (ALC-NIBP, Alcott Biotech, Shanghai, China) before surgery. Recordings were started after 5–10 min of acclimation. Three successful measurements were averaged as a single data point.
Measurement of ventricular arrhythmiasAfter a 10 min stabilization period, Heart rate (HR) was taken as the baseline value using an MP 150 data acquisition and analysis system (BIOPAC Systems Inc., Goleta, CA, USA). Ventricular arrhythmias were recorded from LAD occlusion to 3 h of reperfusion. The severity of arrhythmias in each heart in the vehicle-I/R group, the olmesartan-I/R group, the vehicle-RIPER group and the olmesartan-RIPER group was quantified by a scoring system in which each individual heart was evaluated via a five-point arrhythmia score as previously described 16.
Measurement of serum creatine kinase and left ventricular mass indexAt the end of reperfusion, artery blood samples were collected from the left carotid artery. The serum creatine kinase (CK) of samples was measured using a Creatine Kinase Kit [NAC Method] (Kehua Bio-engineering Co., Ltd, Shanghai, China) and TBA–120FR automatic biochemistry analyzer (Toshiba Corporation, Tokyo, JP) according to the manufacturer's instructions.
After the blood samples were collected, the LAD was re-occluded and 1 ml of 2% Evans blue dye was injected into the right jugular vein to stain the normally perfused region of the heart and delineate the area at risk. Then, the rat was killed by injection of 10% potassium chloride through the right carotid artery. The entire heart was excised and rinsed of excess blue dye. The right ventricle and atria were trimmed off. The weight of the left ventricle (LVW) was measured. Then, the left ventricular mass index (LVMI) was calculated as LVW to BW ratio (LVW/BW, mg/g).
Evaluation of the area at risk and myocardial infarct sizeThe left ventricle was frozen at −80°C. The frozen left ventricle was cut into approximately six sections from the apex to then base, and then, all tissues were incubated in a 1% solution of 2,3,5-triphe-nyltetrazolium chloride (TTC) at 37°C for 15 min to differentiate necrotic (pale) from non-necrotic (red) areas at risk. The slices were compressed between glass plates and scanned into a computer (Epson model G850A, Seiko Epson Corporation, Nagano, Japan). After magnification, planimetry was carried out using image analysis software (Image-Pro Plus 6.0, Media Cybernetics, Rockville, MD, USA). The risk region and infarct region were calculated from surface area analysis. After the summation of individual slices, the area at risk (AAR) was expressed as a percentage of the left ventricle area (AAR/LV) and the myocardial infarct (MI) size was expressed as a percentage of AAR (MI/AAR).
Extraction and analysis of HIF–1α and HIF-related hypoxamirs (miR-21 and miR-210)Total microRNA was extracted from the AAR of myocardial tissue (100 mg) using a UNIQ–10 Column Trizol Total RNA Isolation Kit (Sangon Biotech, Shanghai, China) and reversely transcribed into cDNA using an Avian Myeloblastosis Virus First Strand cDNA Synthesis Kit (Sangon Biotech, Shanghai, China) according to the manufacturer's instructions.
Analysis of gene expression was studied using real-time RT-PCR with a SYBR Green PCR Master Mix (Applied Biosystems Inc., Carlsbad, CA, USA) and the LightCycler 480 Real-Time PCR System (Roche Diagnostics Ltd., Rotkreuz, Switzerland). The reaction program was 95°C for 3 min, followed by 40 cycles at 95°C for 15 s and 60°C for 40 s. GAPDH was used as an internal control for HIF–1α and U6 for HIF-related hypoxamirs (miR-21 and miR-210). The primers for GAPDH, HIF–1α, U6 and HIF-related hypoxamirs (miR-21 and miR-210) are listed in Table 1. The relative expression levels of HIF–1α and HIF-related hypoxamirs were analyzed by the 2-ΔΔCT relative quantification method 17.
Primers used for real-time RT-PCR.
| RT primers | ||
|---|---|---|
| miR-21 | 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAACATC–3′ | |
| miR-210 | 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAGCCGC–3′ | |
| U6 | 5′-AACGCTTCACGAATTTGCGT–3′ | |
| PCR primers | Forward | Reverse |
| HIF-1α | 5′-GACACGGAAACTGAAGACCAA–3′ | 5′-CATTAGAAGAGGGGAACATTACATC–3′ |
| GAPDH | 5′-CAAGTTCAACGGCACAGTCAA–3′ | 5′-CGCCAGTAGACTCCACGACA–3′ |
| miR-21 | 5′-ACACTCCAGCTGGGTAGCTTATCAGACTG–3′ | 5′-TGGTGTCGTGGAGTCG–3′ |
| miR-210 | 5′-ACACTCCAGCTGGGCTGTGCGTGTGACA–3′ | 5′-TGGTGTCGTGGAGTCG-3′ |
| U6 | 5′-CTCGCTTCGGCAGCACA-3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
HIF-1α, hypoxia-inducible factor (HIF) −1α.
miR, microRNA.
RT-PCR, reverse transcription-polymerase chain reaction.
Data are expressed as mean values ± SD. In experiments with more than two conditions, statistical analysis was performed using one-way analysis of variance (ANOVA) with Dunnett's post-hoc analysis. When only two conditions were compared (except for the arrhythmia score), analysis was performed by Student's T-test. Differences in the arrhythmia score between the groups were compared by the Mann-Whitney U-test. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
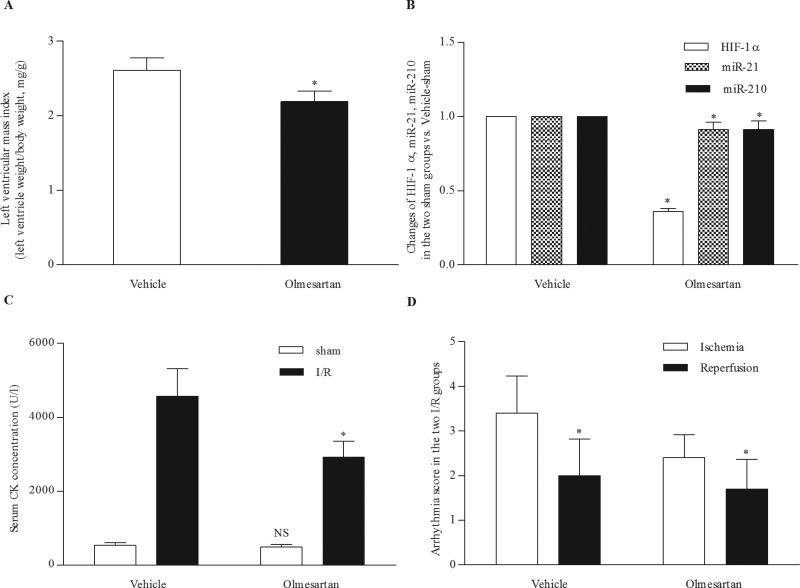
RESULTSChronic treatment with olmesartan reduced cardiac hypertrophy, limited the infarct size and reduced the severity of arrhythmiasOlmesartan reduced BP and ameliorated left ventricular hypertrophy. Four weeks of olmesartan treatment significantly decreased SBP, DBP and LVMI in the olmesartan-treated SHRs (132.5±17.8 mm Hg, 97.40±17.2 mm Hg, and 2.19±0.14, respectively) compared to the vehicle-treated SHRs (183.9±13.3 mm Hg, 130.6±15.9 mm Hg, and 2.61±0.17, respectively) (Figure 1A).
Effect of chronic treatment with olmesartan. (A) Olmesartan markedly reduced the left ventricular mass index. *p<0.001 Olmesartan-treated SHRs versus vehicle-treated SHRs. (B) Olmesartan significantly decreased the expression of HIF-1α, miR-21 and miR-210. *p<0.001 Olmesartan-sham group versus vehicle-sham group. (C) Olmesartan markedly reduced the serum CK concentration in SHRs with I/R injury. NS, p=0.168 Olmesartan-sham group versus vehicle-sham group. *p<0.001 Olmesartan-I/R group versus vehicle-I/R group. (D) Olmesartan markedly decreased the arrhythmia score during ischemia and reperfusion. *p<0.05 Olmesartan-I/R group versus vehicle-I/R group. CK, creatine kinase; HIF-1α, hypoxia-inducible factor-1α; I/R, ischemia/reperfusion; miR, microRNA; SHRs, spontaneously hypertensive rats.
In addition, olmesartan reduced the expression of HIF–1α, miR-21 and miR-210. The expression levels of HIF–1α, miR-21, and miR-210 were significantly lower in the olmesartan-sham group than those in the vehicle-sham group (Figure 1B).
Moreover, myocardial infarct size was significantly decreased in the olmesartan-I/R group (55.6±5.6%) compared to the vehicle-I/R group (67.4±6.3%). Although the CK concentration was comparable between the olmesartan-sham group and vehicle-sham group, it was lower in the olmesartan-I/R group (2919.9±441.6 U/l) than in the vehicle-I/R group (4574.2±744.2 U/l) (Figure 1C).
Furthermore, olmesartan affected the severity of arrhythmias during ischemia and reperfusion, which was evaluated via arrhythmia score. The arrhythmia scores during ischemia and reperfusion were 3.4±0.8 and 2.4±0.5, respectively, in the vehicle-I/R group and 2.0±0.8 and 1.70±0.7, respectively, in the olmesartan-I/R group (Figure 1D).
The protective effect of RIPER was abrogated in the vehicle-treated SHRs but restored in the olmesartan-treated SHRsAs shown in Figure 2A, the AAR was comparable among the four groups. The infarct size was comparable between the vehicle-I/R group and vehicle-RIPER group, whereas it was significantly smaller in the olmesartan-RIPER group (36.4±4.6%) than in the vehicle-I/R, vehicle-RIPER and olmesartan-I/R groups (67.4±6.3%, 64.5±5.1%, 55.6±5.6%, respectively) (Figure 2B).
Effect of chronic treatment with olmesartan and RIPER on the area at risk and infarct size. (A) Area at risk, expressed as a percentage of left ventricle area, was comparable among the four groups. (B) Infarct size, expressed as a percentage of area at risk, was significantly lower in the olmesartan-treated SHRs. NS p=0.286 Vehicle-RIPER group versus vehicle-I/R group. # p<0.001 Olmesartan-I/R group versus vehicle-I/R group. *p<0.001 Olmesartan-RIPER group versus Olmesartan-I/R group. I/R, ischemia reperfusion; RIPER, remote ischemic perconditioning; SHR, spontaneously hypertensive rat.
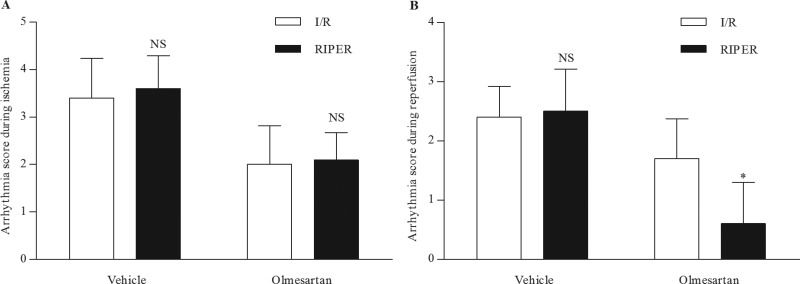
RIPER did not markedly affect the severity of arrhythmias during ischemia (Figure 3A). In the vehicle-treated SHRs, the arrhythmia score during ischemia was comparable in the vehicle-I/R group (3.4±0.8) and vehicle-RIPER group (3.6±0.7). In the olmesartan-treated SHRs, the arrhythmia score during ischemia in the olmesartan-I/R group was 2.0±0.8, which is similar to that in the olmesartan-RIPER group (2.1±0.6).
Effect of RIPER on arrhythmia scores during ischemia and reperfusion. (A) RIPER had no effect on the severity of arrhythmias during ischemia. NS p>0.05 RIPER group versus I/R group. (B) RIPER had no effect on the severity of arrhythmias during reperfusion in vehicle-treated SHRs but significantly reduced the severity of arrhythmias during reperfusion in olmesartan-treated SHRs. NS p=0.796 Vehicle-RIPER group versus vehicle-I/R group. *p<0.01 Olmesartan-RIPER group versus Olmesartan-I/R group. I/R, ischemia reperfusion; RIPER, remote ischemic perconditioning; SHR, spontaneously hypertensive rat.
RIPER did not affect the severity of arrhythmias during reperfusion in the vehicle-treated SHRs but reduced the arrhythmia score during reperfusion markedly in the olmesartan-treated SHRs (Figure 3 B). In the vehicle-treated SHRs, the arrhythmia score during reperfusion was comparable between the vehicle-I/R group (2.4±0.5) and vehicle-RIPER group (2.5±0.7). In the olmesartan-treated SHRs, the arrhythmia score in the olmesartan-I/R group was 1.7±0.7, which was markedly attenuated to 0.6±0.7 in the olmesartan-RIPER group.
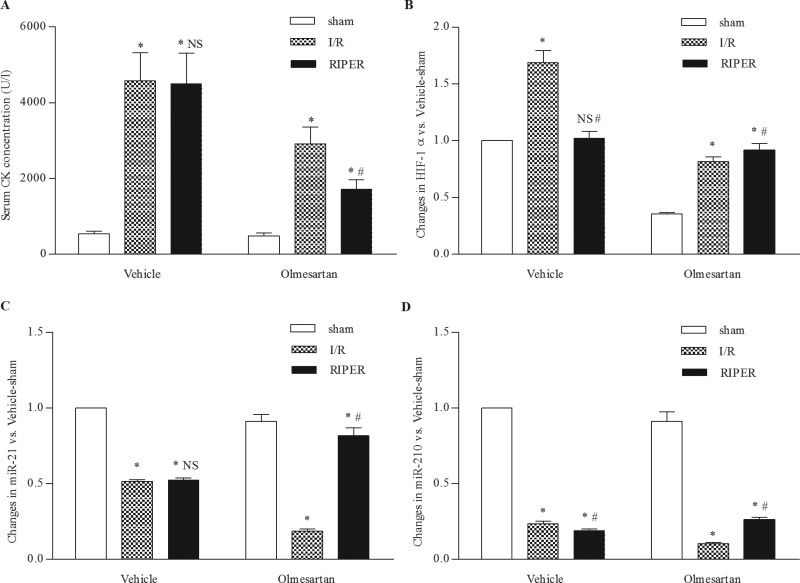
RIPER did not have a significant influence on CK concentration in the vehicle-treated SHRs but caused a significant reduction in CK concentration in the olmesartan-treated SHRs. There was no significant difference in the CK concentration between the vehicle-I/R group (4574.2±744.2 U/l) and vehicle-RIPER group (4,503.2±806.6 U/l) in the vehicle-treated SHRs. However, the CK concentration was significantly lower in the olmesartan-RIPER group (1,710.3±252.9 U/l) than in the olmesartan-I/R group (2,919.9±441.6 U/l) in the olmesartan-treated SHRs (Figure 4A).
Effect of RIPER on SHRs with I/R injury. (A) I/R injury led to a significant increase in the serum CK concentration. RIPER markedly decreased the serum CK concentration in olmesartan-treated SHRs, whereas the serum CK concentration is similar for vehicle-treated SHRs. *p<0.001 versus sham group. #p<0.001 versus I/R group. NS p=0.996 versus I/R group. (B) I/R injury significantly increased HIF-1α expression. Compared to the vehicle-I/R group, HIF-1α expression was significantly decreased in the vehicle-RIPER group. Compared to the olmesartan-I/R group, HIF-1α expression was significantly increased in the olmesartan-RIPER group. *p<0.001 versus sham group. NS p=0.585 versus sham group. #p<0.001 versus I/R group. (C) I/R injury markedly down-regulated miR-21 expression. miR-21 expression was comparable between the vehicle-I/R group and vehicle-RIPER group, whereas it was significantly up-regulated in the olmesartan-RIPER group compared to the olmesartan-I/R group. *p<0.01 versus sham group. #p<0.001 versus I/R group. NS p=0.886 versus I/R group. (D) I/R injury significantly down-regulated miR-210 expression. miR-210 expression was significantly down-regulated in the vehicle-RIPER group compared to the vehicle-I/R group, whereas it was significantly up-regulated in the olmesartan-RIPER group compared to the olmesartan-I/R group. *p<0.001 versus sham group. #p<0.01 versus I/R group. HIF-1α, hypoxia-inducible factor-1α; I/R, ischemia reperfusion; miR, microRNA; RIPER, remote ischemic perconditioning; SHR, spontaneously hypertensive rat.
I/R injury caused significant up-regulation in the mRNA expression level of HIF–1α and significant down-regulation in the expression level of miR-21 and miR-210. In the olmesartan-treated SHRs, the expression level of HIF–1α was significantly increased in the olmesartan-RIPER group compared to the olmesartan-I/R group (Figure 4B). The expression levels of miR-21 and miR-210 were also up-regulated in the olmesartan-RIPER group compared to the olmesartan-I/R group in the olmesartan-treated SHRs (Figures 4C and 4D). In the vehicle-treated SHRs, the expression level of HIF–1α was significantly down-regulated in the vehicle-RIPER group compared to the vehicle-I/R group. Specifically, the expression level of HIF–1α was comparable between the vehicle-sham group and vehicle-RIPER group. The expression level of miR-21 in the vehicle-RIPER group is similar to that in the vehicle-I/R group. The expression level of miR-210 is significantly lower in the vehicle-RIPER group than in the vehicle-I/R group.
DISCUSSIONIn this study, we demonstrate that (i) the protective effect of RIPER was lost in SHRs and chronic treatment with olmesartan restored the protective effect of RIPER in SHRs; (ii) chronic treatment with olmesartan reduced I/R injury in SHRs and RIPER further reduced I/R injury in olmesartan-treated SHRs; and (iii) HIF–1α, miR-21 and miR-210 were involved in the protection provided by olmesartan and RIPER.
Cardiac hypertrophy occurring in response to hypertension is an independent and major risk factor for cardiovascular morbidity and mortality 18. In SHRs, increased oxidative stress characterized by increased ROS-generating capacity contributes to the overexpression of HIF-1α, which may be protective during the stage of compensatory hypertrophy but may eventually contribute to the pathology of end-stage failure 19. In addition, miR-210, a hypoxia-specific microRNA induced by HIF-1α, has been found to be a key factor regulating HIF-dependent control of the Pasteur effect via its repression of mitochondrial metabolism in multiple cell types exposed to hypoxia 20. Moreover, miR-21, a highly expressed microRNA in the cardiovascular system, is up-regulated in the heart during pathological hypertrophy 21.
In our experiment, four weeks of olmesartan treatment significantly reduced left ventricular hypertrophy. The expression of HIF-1α, miR-21 and miR-210 was significantly reduced in the olmesartan-sham group compared to the vehicle-sham group. The result indicated that olmesartan reduced oxidative stress by inhibiting the AT1 receptor, thereby reducing the expression of HIF-1α, miR-210 and miR-21. Fu demonstrated that olmesartan attenuated cardiac hypertrophy in SHRs via the calcineurin pathway 22, whereas our study proved that the beneficial effect of olmesartan on reducing cardiac hypertrophy was due to the down-regulation of expression of HIF-1α, miR-210, and miR-21.
I/R injury is a pathophysiologic process whereby hypoxic tissue damage is accentuated following restoration of the blood supply. Hypertrophied myocardium is at a greater risk of sustaining injury and electrophysiological disturbances after I/R 23. In our experiment, four weeks of olmesartan treatment significantly reduced the serum CK concentration, attenuated the severity of arrhythmias and limited the infarct size in the olmesartan-I/R group compared to the vehicle-I/R group. Dai et al. 24 reported that acute treatment with olmesartan given at 5 min before the reperfusion significantly reduced the myocardial infarct size and improved left ventricular contractility; this result may be related to the vasodilating effect observed with the higher dose of olmesartan with subsequent decrease in oxygen demand. In our experiment, we proved that olmesartan benefited I/R myocardium in SHRs by ameliorating left ventricular hypertrophy.
RIPER was first reported in the experimental studies of Schmidt et al. 1 and later in the clinical studies of Botker et al. 25. Schmidt et al. 1 proved that intermittent limb ischemia during myocardial ischemia reduced the myocardial infarct size, preserved the global systolic and diastolic functions, and protected against arrhythmia during the reperfusion phase through a KATP channel-dependent mechanism. In our experiment, the protection afforded by RIPER was abrogated in vehicle-treated SHRs. The myocardial infarct size, serum CK concentration and severity of arrhythmias were comparable between the vehicle-I/R group and vehicle-RIPER group.
However, chronic treatment with olmesartan restored the protective effect of RIPER in olmesartan-treated SHRs. The myocardial infarct size and serum CK concentration were significantly lower in the olmesartan-RIPER group than in the olmesartan-I/R group. Although the arrhythmia score during ischemia was comparable between the olmesartan-RIPER group and olmesartan-I/R group, the arrhythmia score during reperfusion was significantly lower in the olmesartan-RIPER group than in the olmesartan-I/R group.
Compared to the olmesartan-I/R group, RIPER increased the expression of HIF-1α in the olmesartan-RIPER group. Activation of HIF-1α and its downstream target genes, including erythropoietin, hemeoxygenase-1, adiponectin and inducible nitric oxide synthase, during ischemia is associated with improved myocardial tolerance to acute I/R injury 26. Studies have also demonstrated that HIF-1α is involved in cardioprotection by IPC 4 and IPOST 5. Our study demonstrates that four weeks of olmesartan treatment changes the effect of RIPER on HIF-1α mRNA expression; the recovery of the effect of RIPER may be partially due to the up-regulation of HIF-1α.
miR-210 and miR-21 are two HIF-1α related hypoxamirs that have emerged as regulators of I/R injury. miR-210, as the master hypoxamir, regulates several angiogenic factors, inhibits caspase activity and protects against apoptosis in both primary and transformed cells 27. Furthermore, the down-regulation of miR-210 increases ROS levels after hypoxia-reoxygenation 28. In our experiment, the expression of miR-210 was up-regulated in the olmesartan-RIPER group compared to the olmesartan-I/R group. Kim found that miR-210 is a regulator of the cytoprotection afforded by IPC in stem cells 29, whereas our experiment shows that the recovery of the protective effect of RIPER may be partially due to the up-regulation of miR-210. miR-21 is also a critical regulator of cell apoptosis. By targeting the Fas ligand 30 and programmed cell death 4 31, exogenous miR-21 assumes a powerful anti-apoptotic function. Specifically, miR-21 is acutely reduced within the ischemic region during ischemia, where replenishing it reduces the infarct size 32. Our experiment shows that the expression of miR-21 was up-regulated in the olmesartan-RIPER group compared to the olmesartan-I/R group. Tu 33 demonstrated that miR-21 was remarkably up-regulated in mouse hearts after IPOST, and the protection provide by IPOST may be related to the anti-apoptotic role of miR-21. Our experiment demonstrates that the recovery of the protection afforded by RIPER may be partially due to the up-regulation of miR-21.
We demonstrate for the first time that the protective effect of RIPER is lost in vehicle-treated SHRs and that chronic treatment with olmesartan restores the protective effect of RIPER in SHRs. Moreover, chronic treatment with olmesartan down-regulates the expression of HIF-1α, miR-21, and miR-210 and thereby reduces left ventricular hypertrophy. Due to the ameliorated hypertrophy, olmesartan reduces the CK concentration, limits the infarct size and decreases the severity of arrhythmias in SHRs with I/R injury. Furthermore, RIPER further reduces the CK concentration, reduces the infarct size and decreases the severity of arrhythmias during reperfusion after chronic treatment with olmesartan. Chronic olmesartan treatment changes the effect of RIPER on HIF-1α, miR-21 and miR-210 expression. The recovery of the protective effect of RIPER may be related to the up-regulation of the expression of HIF-1α, miR-21 and miR-210.
Compared to other conditioning techniques, RIPER is a noninvasive procedure and does not prolong the operating time. However, the protective effect of this easy-to-use, low-risk procedure is abrogated in SHRs. Systemic hypertension is a common disorder worldwide. Hypertension-related clinical sequels, such as ischemic heart disease and myocardial infarction, continue to present pressing challenges to the healthcare system. Olmesartan is a new ARB that is increasingly used to treat hypertension. Our study demonstrates that chronic treatment with olmesartan not only reduces I/R injury but also restores the protective effect of RIPER in SHRs. Therefore, olmesartan may provide a greater long-term benefit for hypertensive patients with coronary artery disease.
Our experiment has demonstrated that chronic olmesartan treatment changes the effect of RIPER on the expression of HIF-1α, miR-21 and miR-210. The recovery of the protective effect of RIPER may be related to up-regulation of the expression of HIF-1α, miR-21 and miR-210. However, whether RIPER can directly up-regulate the expression of HIF-1α, miR-21 and miR-210 remains unclear. Future research should attempt to determine whether up-regulation of the expression of HIF-1α, miR-21 and miR-210 is required for the protective effect afforded by RIPER.
AUTHOR CONTRIBUTIONSLu X participated in the experimental design, carried out the experiments, analyzed the data, prepared the figures and wrote the first draft of the manuscript. Bi YW participated in the experimental design, reviewed the manuscript and approved the final version of the manuscript. Chen KB performed part of the experiments and conducted the literature search.