Lung transplantation is a well-established treatment for advanced lung diseases. In children, the diseases that most commonly lead to the need for a transplantation are cystic fibrosis, pulmonary hypertension, and bronchiolitis. However, the number of pediatric lung transplantations being performed is low compared with the number of transplants performed in the adult age group. The objective of this study was to demonstrate our experience with pediatric lung transplants over a 10-year period in a program initially designed for adults.
Lung transplantation (LTx) is now a well-established therapy for treating various chronic lung diseases that lead to severe respiratory failure. LTx has improved significantly since it began in 1963 with James Hardy (1). An increasing number of studies have focused on studying the indications, techniques, immunosuppressive drugs, and criteria for donation. The first pediatric LTx occurred in 1987 in a 16-year-old boy with familial pulmonary fibrosis (2).
The annual number of pediatric lung transplants performed is significantly lower than that of adult transplant surgeries performed each year. According to a 2013 report from the International Society of Heart and Lung Transplantation, from 1986 to June 2012, in patients younger than 18 years, 1875 lung transplants and 667 double heart and lung transplantations were reported (3), whereas there were 3640 transplanted adults in 2011 (4).
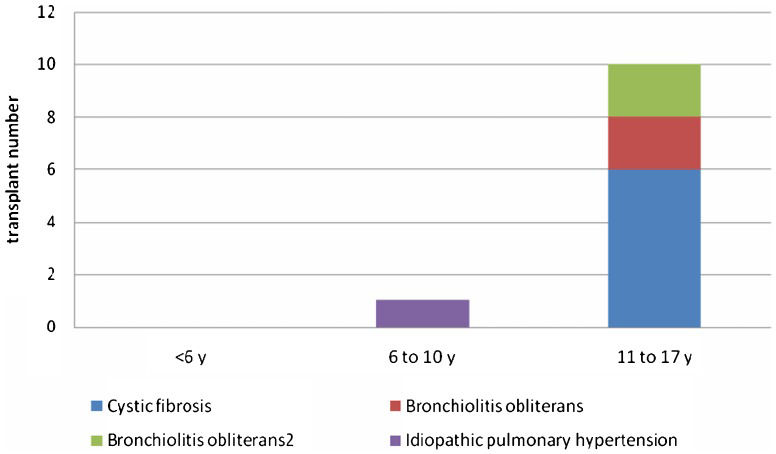
The most common indication for LTx in patients up to 18 years old is cystic fibrosis (3), a fact that is also demonstrated in this study. Other indications for LTx include bronchiolitis obliterans, bronchiectasis, pulmonary arterial hypertension, idiopathic fibrosis, interstitial lung diseases, and surfactant protein abnormalities (5).
The aim of this study was to describe the pediatric lung transplantation experiences at the Heart Institute (InCor) of Faculdade de Medicina da Universidade de São Paulo.
PATIENTS AND METHODSA retrospective analysis of the medical records of patients undergoing lung transplantation at the Heart Institute (Incor) of Hospital das Clínicas da Faculdade de Medicina de São Paulo was performed for the period from January 2003 to October 2013.
RESULTSFrom February 2003 to October 2013, 192 lung transplants were performed at our institution (11 patients 18 years old and younger). The recipient distribution by age and diagnosis is shown in Figure1.
All 11 pediatric patients underwent bilateral sequential LTx; two patients required cardiopulmonary bypass during surgery. To date, the longest surviving pediatric transplant patient underwent surgery 5 years ago when he was 11 years old.
Two patients suffered immediate postoperative death less than one month post transplantation. The first patient, whose initial diagnosis was idiopathic pulmonary arterial hypertension, developed primary graft dysfunction and died on the 4th postoperative day. This patient was six years old and required cardiopulmonary bypass. The second patient, a 17-year-old adolescent diagnosed with bronchiolitis obliterans associated with secondary pulmonary hypertension, also underwent cardiopulmonary bypass; the patient developed refractory hemodynamic instability and died three days post-surgery.
One patient with an initial diagnosis of bronchiectasis died three years after undergoing transplantation for chronic graft dysfunction with clinical restrictive syndrome. A 16-year-old patient with an initial diagnosis of post-infectious bronchiolitis obliterans syndrome later developed obliterative bronchiolitis and underwent retransplantation three years after the initial procedure.
In our institution, induction therapy is performed with 10 mg/kg intravenous methylprednisolone, with basiliximab in the case of initial suppurative disease. The maintenance immunosuppression combines corticosteroids (prednisone), calcineurin inhibitor (cyclosporine or tacrolimus), and a cellular activation inhibitor (azathioprine or mycophenolate sodium).
The complications related to the immunosuppressive regimen were infection (in the majority of cases) and reversible posterior leukoencephalopathy (one case) in a patient initially diagnosed with cystic fibrosis. This patient was on cyclosporine and showed neurological symptoms eight days after the procedure, progressing to acute subdural hemorrhage. Currently, he has motor neurological deficit without other complications.
In our study, the most common infection found after the first month of transplant was due to citomegalovirus (CMV), followed by respiratory viral infections and bacterial infections.
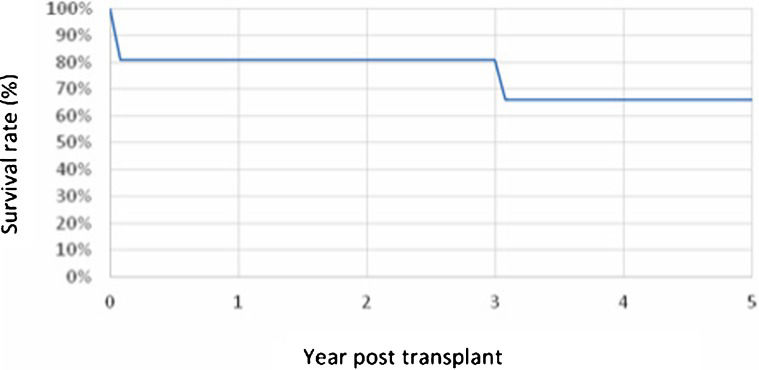
Figure2 shows the survival rate based on the time after transplantation for patients 18 years old and younger.
DISCUSSIONLTx is a well-established therapy for patients with chronic lung disease and end-stage vascular pulmonary disease. Indications for LTx in the pediatric population are rare because terminal illnesses in this age group are uncommon (5). Most patients younger than 18 years who undergo lung transplantation are older than 11 years (3,6), which was also found in our study group.
The main indications for LTx in the pediatric population are pulmonary cystic fibrosis, interstitial lung disease associated with pulmonary fibrosis, and congenital vascular disease (4,5). Furthermore, in Brazil, the prevalence of bronchiectasis (not secondary to cystic fibrosis) is considerable, and this condition frequently requires LTx.
In the adult population, the main indications are emphysema and idiopathic pulmonary fibrosis (4).
The pediatric contraindications for the procedure are identical to those in the adult population and include systemic diseases with extrapulmonary complications, malignancy, HIV infection, and severe renal impairment. The absence of social support, transportation issues, or poor adherence to treatment are also important conditions that preclude LTx (7).
The number of pediatric transplants performed in our study group, although small, corresponded to approximately 5.7% of the total number of transplants performed, which did not differ from the literature (3). The survival rate was also similar to that of the international registry, but a longer follow-up is necessary, as illustrated by the patient with a longer follow-up who underwent surgery 5 years earlier.
The low number of donors and viable organs for donation is a significant complicating factor that has affected the pediatric and adult LTx program.
As in the adult population, there are three main types of postoperative complications: immediate complications, occurring in the first days after transplantation; early complications, occurring within three months posttransplantation; and late complications (8).
The immediate postoperative complications are hyperacute rejection, primary graft dysfunction, reperfusion injury, and surgical complications, such as anastomotic dehiscence and bleeding.
Hyperacute rejection is an uncommon condition. Today, virtual crossmatching performed preoperatively in patients with a high reactive panel identifies any possible incompatibility that could preclude transplantation between the recipient and donor. Hyperacute rejection has a poor prognosis, and treatment should be started immediately if the prospective crossmatch is positive. The treatment consists of plasmapheresis in the operating room and during the postoperative period, with the administration of intravenous immunoglobulin and thymoglobulin as an additional potential indication for retransplantation (8,9).
Another important complication is primary graft dysfunction, which occurred in our setting and can occur within 72 hours post-surgery. Primary graft dysfunction is defined by a low relationship between PaO2 and FiO2, with or without radiographic changes. The complication is described in Table1. International data show that cardiopulmonary bypass is used more frequently in pediatric LTx compared with adult LTx, and its use is related to the development of primary graft dysfunction, smoking status of the donor, presence of reperfusion injury, and body mass index of the recipient (8,10,11). In acute respiratory distress syndrome, clinical management consists of protective ventilation and, in some cases, the use of extracorporeal membrane oxygenation (10,12,13).
Classification of Primary graft dysfunction - Adapted from ISHLT (International Society of Heart and Lung Transplantation Registries) (10).
| Grades | PaO2/FiO2 | Persistant pulmonary infiltration at radiographic image with pulmonary edema |
|---|---|---|
| 0 | >300 | Absence |
| 1 | >300 | Present |
| 2 | 200-300 | Present |
| 3 | <200 | Present |
Acute rejection, infection, side effects of immunosuppressive drugs, and surgical complications, such as stenosis of the bronchial anastomosis, are some immediate postoperative complications; they frequently occur in the first three months after LTx.
Clinically, diagnosing acute rejection and respiratory infections can be challenging, and they require different treatments. The diagnosis of acute rejection is made using histological findings that show perivascular mononuclear cell infiltrate. The intensity and extent of the acute rejection determines its classification (14). When indicated, treatment consists of adjusting the immunosuppression, including changing or increasing the dose of the immunosuppressive maintenance regimen, administering high doses of corticosteroids (in some cases), and using intravenous thymoglobulin in more severe or refractory cases (15). The infection treatment is based on etiological agents.
Infections are often related to the use of immunosuppressive drugs, and infections are one type of complication that can be present during the entire posttransplant follow-up period. The use of maintenance medications has other undesirable effects, including metabolic changes (hyperglycemia and dyslipidemia) and nephrotoxicity. In our study group, one patient had reversible posterior leukoencephalopathy, a disease that is often associated with the use of calcineurin inhibitors and that has variable outcomes (16,17). It involves a wide spectrum of neurological manifestations, such as a decreased level of consciousness, seizures, and visual disturbances that are frequently associated with ischemic changes mainly in parietal and occipital lesions, as evidenced by MRI. The reversibility of lesions is case dependent. Patient management usually consists of reducing or changing the calcineurin inhibitor. Our case showed reversibility of the changes after the initial change in the immunosuppressant agent, but the patient developed hemorrhagic transformation and persistent motor deficiency.
Bronchiolitis obliterans syndrome is the main late complication; it corresponds to chronic graft dysfunction. Bronchiolitis obliterans syndrome is defined by a 20% drop in the forced expiratory volume (FEV1) value in one second compared with the patient's baseline, and it is diagnosed using the best three FEV1 values previously taken after the development of fixed airflow obstruction on spirometry and in the absence other factors that may influence the collection method (18). The differential diagnosis includes other causes of FEV1 decline, such as anastomotic stenosis or respiratory infections. In some cases, the patient may present with severe dysfunction and end-stage pulmonary disease, and any indication for retransplantation should be carefully evaluated (5). In our study group, retransplantation was indicated three years after the first procedure in a patient who underwent transplantation at age 16.
The indications for LTx in a pediatric population are less common than those in an adult population. Certain peculiarities, such as the prevalence of different diseases, indicate the procedure and direct its perioperative management, although the postoperative complications and treatments are similar to those found in patients older than 18. In our setting, the indications, prevalence of pediatric LTx, and survival rates were similar to those in the international data, despite the small number of patients.
AUTHOR CONTRIBUTIONSCamargo PC and Pato EZ wrote the manuscript and collected the data. Campos SV, Afonso Jr JE, Carraro RM, and Costa NA collected the data. Teixeira RH and Pego-Fernandes PM revised the manuscript. Samano MN wrote and revised the manuscript and collected the data.
No potential conflict of interest was reported.