Pusher behavior (PB) is a postural control disorder characterized by actively pushing away from the nonparetic side and resisting passive correction with a tendency to fall toward the paralyzed side.1 These patients have no awareness that their active pushing is counterproductive, which precludes the patients from standing without assistance.
Several studies have already demonstrated that PB can occur in patients with lesions in both hemispheres, and PB is distinct from neglect and anosognosia.2–8 The high frequency of the association between PB and neurophysiological deficits might reflect an increased vulnerability of certain regions to stroke-induced injury rather than any direct involvement with the occurrence of PB.9,10
Traditionally, PB has only been reported in stroke patients; however, it has also been described under non-stroke conditions.8 Previous imaging studies have suggested the posterolateral thalamus as the brain structure that is typically damaged in pusher patients.4,11 Nevertheless, other cortical and subcortical areas, such as the insular cortex and post-central gyrus, have also been highlighted as structures that are potentially involved in the pathophysiology of PB.2,12–16
The mechanisms underlying PB have been attributed to vertical perception dysfunction that leads to postural reactive behavior.3,14,17–19 Nevertheless, the true changes in the verticality perception of these patients are still unclear. In this context, Karnath et al. identified five patients with severe PB who experience their body (subjective postural vertical [SPV]) as oriented “upright” when it is actually tilted approximately 18° toward the side of the brain lesion and with no subjective visual vertical (SVV) bias.3 Johansen et al. also found no SVV bias in 15 PB patients.20 In contrast, Pérrenou et al. found SPV, SVV, and subjective haptic vertical (SHV) biases toward the side opposite the brain lesion in six pusher patients.21 It is clear that, to state which vertical perception is disturbed in PB patients, the studies' designs require a meticulous methodology, including the analysis of neglect and the influence of haptics on SVV in a large sample of PB patients.
Until now, PB has only been reported as a temporary and transitory phenomenon with a maximum recovery time of six months.8,22–24 Moreover, it has been suggested that PB does not negatively influence the functional outcomes of rehabilitation.10,22 Nevertheless, those assumptions have primarily emerged from case series of stroke patients admitted to stroke units or followed in rehabilitation centers in developed countries.10,22–24 Therefore, the actual impact of the disorder on stroke patients in developing countries may be underestimated. Here, we report three cases of stroke patients that had persistent PB with important disabling consequences on their functional outcomes.
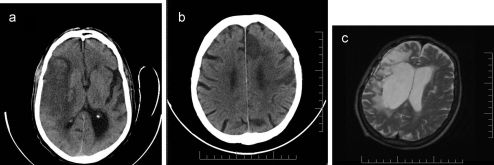
CASE DESCRIPTIONPatient 1: A 77-year-old right-handed male with a history of hypertension was admitted after being found confused and left-hemiparetic with an NIH stroke scale25 score of 20. Cranial CT revealed a right-middle cerebral artery ischemic stroke (Figure 1a; Table 1). The PB was identified 10 days after the onset of ictus with a Scale for Contraversive Pushing (SCP)3 score of 6, and he was discharged 10 days later. The patient was reevaluated 318 days after the onset of ictus and still presented severe PB (SCP = 6) and a Barthel Index25 score of zero. He died because of pneumonia a few weeks after his last reevaluation.
a. CT scan from patient 1. Note an ischemic stroke of the M1 segment of the middle cerebral artery with a small hemorrhagic transformation in the perforating arteries territory. b. CT scan from patient 2 showing infarcts in branches of left anterior and middle cerebral arteries. c. MRI scan from patient 3 with an area of right frontotemporal encephalomalacia, 1.6 years after the surgical clipping of a right middle cerebral artery aneurysm.
The demographic and clinical data of the patients.
| Patient | Recovery time | NIHSS | SCP | Neglect | Anosognosia | Sensitive deficit | Muscle strengh Contralesioinal limbs/Ipsilesional limbs | Hemianopia | Aphasia | Dysarthria | Previous encephalic lesion | Barthel Index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 318 | 20 | 6 | Yes | Yes | Hypoesthesia | 0/5 | Yes | No | Yes | No | 0 |
| 2 | 763 | 21 | 6 | No | No | Hypoesthesia | 1/5 | Yes | Yes | Yes | No | 0 |
| 3 | 789 | 14 | 6 | Yes | No | Hypoesthesia | 2/4 | Yes | No | Yes | No | 10 |
Patient 2: A 74-year-old right-handed male with a history of alcohol and tobacco abuse was admitted with right hemiparesis and aphasia. A CT scan revealed ischemic strokes in the branches of his left-anterior and middle cerebral arteries (Figure 1b; Table 1). PB was identified nine days after the onset of ictus (SCP = 6). He was discharged after 20 days. Upon his last reevaluation 763 days after the onset of ictus, severe PB was still present, and he had a Barthel Index score of zero.
Patient 3: A 65-year-old right-handed man with a history of a ruptured right-middle cerebral artery aneurysm treated by surgical clipping in another institution was referred to our outpatient stroke clinic 1.6 years after the onset of ictus when severe PB was identified (Figure 1c; Table 1). Severe PB was still present 729 days after the onset of ictus, and he had a Barthel Index score of 10. (The patient had occasional bladder and bowel incontinence.) He was found dead by his family members a few weeks after his final evaluation.
In all of the cases, after the identification of PB, the patients were referred to public rehabilitation centers, but their adherence to their rehabilitation programs was neither optimized nor controlled. All of the patients had restricted access to physiotherapy (the mean number of sessions per week was less than 1) and remained lying in bed almost the entire day. Furthermore, upon their final reevaluations, all of the patients were completely dependent on their activities of daily living with a modified Rankin scale score of 5, the strengths of their arms and legs opposite their brain lesions did not change over time, and they had a persistent fear of falling.
DISCUSSIONThis is the first demonstration that PB can persist for more than two years, with disastrous impacts on functional outcomes. Certainly, the factors that negatively influenced the duration of PB were the limited frequency of physiotherapy and the absence of specific strategies for treating PB. In fact, some well-described physiotherapy approaches have been proposed,23,26–29 but there have been no randomized control trials that have confirmed their effectiveness. Still, there is strong evidence that stroke rehabilitation that is initiated early after onset and sustained across the healthcare continuum significantly reduces the probability of disability within the first year.30,31 Furthermore, the poor socioeconomic conditions of the patients restricted their home care and led to the patients lying in bed almost the entire day, despite their physiotherapeutic recommendations. Thus, the privation of experience in the vertical position after their strokes could have negatively influenced the patients' prognoses. This observation raises the question of whether the duration of PB could be shortened by the simple attitude of remaining in a vertical position for several daylight hours.
PB has been considered to be a neurological behavior that is not strongly associated with the recovery of motor control in the upper and lower limbs.24,32 Nevertheless, we observed poor concomitant PB recovery and other neurological deficits, which might reflect the maladaptive mechanisms underlying the lack of plasticity. Additional studies will be needed to understand the neuroplastic mechanisms of PB recovery using brain-activation techniques (functional magnetic resonance, single-photon emission computed tomography, positron emission tomography, magnetoencephalography, and event-related potential) and transcranial magnetic stimulation techniques.
Spatial neglect has been identified as a factor that worsens the prognosis of PB,6,10,24 and this clinical characteristic was found in two of our patients. Thus, we cannot suggest that the poor recovery observed in our patients could be entirely due to the presence of spatial neglect. Moreover, we were unable to evaluate the perception of verticality. Future studies should explore whether the vertical misperceptions of the pusher patients influence the duration and severity of PB.
The absence of sitting balance soon after stroke has consistently been shown to predict a poor recovery.33 Moreover, predicting the outcome of a stroke is important for triage decisions, prognostic estimates for the family, and the appropriate utilization of resources.34 It is noteworthy that while the patients presented PB, all of their daily life activities were compromised. Thus, this study highlights that PB can be much more incapacitating than generally believed.
The authors acknowledge CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for their financial support.
No potential conflict of interest was reported.
Santos-Pontelli TEG and Pontes-Neto designed and conceived the study, collected, analyzed, interpreted the data, and drafted the manuscript. Araujo DB and Santos AC designed the study, analyzed, interpreted the data and revised the manuscript. Leite JP designed and conceived the study, analyzed and interpreted the data, and revised the manuscript.