Ischemic preconditioning and some drugs can protect tissues from injury by preserving microcirculation. This study evaluated vascular permeability in a hamster cheek pouch preparation using either short ischemic periods or bradykinin as preconditioning stimuli followed by 30 min of ischemia/reperfusion.
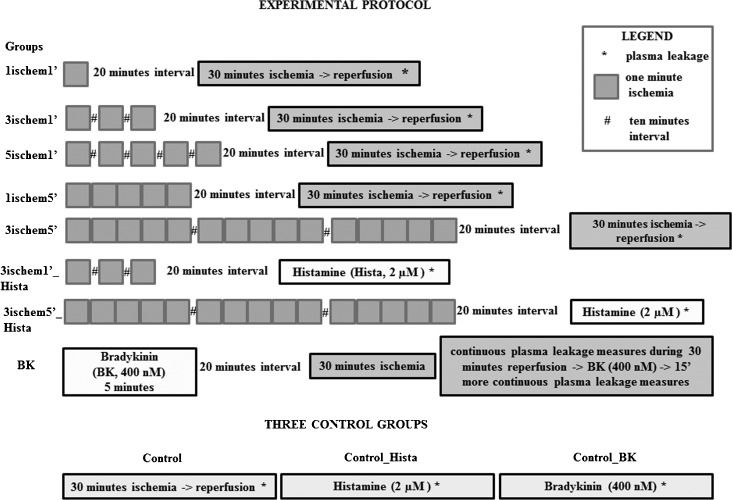
METHOD:Sixty-six male hamsters were divided into 11 groups: five combinations of different ischemic frequencies and durations (one, three or five shorts periods of ischemia, separated by one or five minutes) with 10 min intervals between the ischemic periods, followed by 30 min ischemia/reperfusion; three or five 1 min ischemic periods with 10 min intervals between them followed by the topical application of histamine (2 µM); bradykinin (400 nM) followed by 30 min of ischemia/reperfusion; and three control groups (30 min of ischemia/reperfusion or histamine or bradykinin by themselves). Macromolecular permeability was assessed by injection of fluorescein-labeled dextran (FITC-dextran, MW = 150 kDa; 250 mg/Kg body weight), and the number of leaks/cm2 was counted using an intravital microscope and fluorescent light in the cheek pouch.
RESULTS:Plasma leakage (number of leaks/cm2) was significantly reduced by preconditioning with three and five 1 min ischemic periods, one and three 5 min ischemic periods and by bradykinin. Histamine-induced macromolecular permeability was also reduced after three periods of 5 min of ischemia.
CONCLUSION:Short ischemic periods and bradykinin can function as preconditioning stimuli of the ischemia/reperfusion response in the hamster cheek pouch microcirculation. Short ischemic periods also reduced histamine-induced macromolecular permeability.
Mortality by cardiovascular disease is still an important concern worldwide. According to World Health Organization (WHO), in 2004, coronary heart disease was the second most common cause of death in low- and middle-income countries and the most common cause of death in high-income ones (1). During an acute myocardial infarction, many attempts to reestablish blood flow are performed by physicians to save as many cardiomyocytes as possible before they are damaged by ischemia. However, after blood flow normalization, reperfusion injury also restricts cell survival. Consequently, larger myocardial infarct areas predict worse prognosis due to the loss of contractile mass and inadequate ventricle remodeling through hypertrophy, eventually causing heart failure (2).
Preconditioning refers to a phenomenon wherein tissues are rendered resistant to the deleterious effects of prolonged ischemia and reperfusion by prior exposure to oxidants, brief periods of vascular occlusion, endotoxin derivatives, heat shock, a variety of pharmacological agents (chemical or pharmacological preconditioning), or chronic ethanol consumption (3). Of these perturbations, our best understanding of the mechanisms involved in conferring protection is related to ischemic preconditioning (IPC) (4).
Myocardial preconditioning has been developed to minimize the effects of acute ischemia to the heart. In 1986, Murry et al. reported that four brief episodes of 5 minutes of ischemia in the circumflex coronary in dogs followed by 5 minutes of reperfusion reduced the infarct size by 75% compared to controls (5). Since the publication of that report, many studies have tried to explain the mechanistic steps involved in the preconditioning process, such as mitochondrial ATP-sensitive K+ channels, nitric oxide, adenosine and bradykinin (BK) (6-8).
Several studies in the hamster cheek pouch (HCP) on experimental perturbations with drugs and the subsequent responses suggest that it could be a useful model for studies of IPC and other types of preconditioning (9-12). In a study of ischemia/reperfusion (I/R) in the HCP, a period of 30 minutes of ischemia resulted in a 30% reduction in plasma leakage in response to a second ischemic period, although the response to histamine appeared unchanged (12).
The main element affected by ischemia that determines cell survival is the microcirculation, the smallest vessels of the vascular system; the endothelium plays a key role in the ischemic process. Endothelial barrier dysfunction is a well-recognized response to ischemia, which involves inflammation (13). Many changes occur in the structure of the endothelial monolayer; one example is the widening of paracellular junctions caused by the dissociation of junctional proteins or cytoskeletal contractions (14,15). Increased microvascular permeability results in edema and organ failure. The magnitude of albumin leakage from post-ischemic venules during ischemia-reperfusion injury was reportedly proportional to the number of adherent and emigrated leucocytes (16). However, histamine, normally associated with allergic reactions and increases in vascular permeability through H2 action, has been shown to decrease inflammation by reducing chemotactic responsiveness of leukocytes (17,18). Adachi et al. demonstrated that diphenhydramine, a histamine H1 receptor antagonist, inhibits histamine-N-methyltransferase, which is a histamine-inactivating enzyme in the brain. Thus, brain histamine ameliorated reperfusion injury after cerebral ischemia in rats (19). The combination of biogenic amines such as histamine, bradykinin and ischemic preconditioning may minimize the deleterious effects of ischemia-reperfusion injury on the microcirculation.
The main objectives of this work were to test the use of the hamster cheek pouch as a model of preconditioning and the effects of different frequencies and durations of brief ischemic preconditioning (IP) episodes followed by 30 minutes of total ischemia and reperfusion or histamine compared to the topical application of bradykinin followed by ischemia/reperfusion on microvascular permeability in hamster cheek pouch preparations.
MATERIALS AND METHODSThis study was approved by the Ethical Committee of the State University of Rio de Janeiro, RJ, Brazil (CEA/215/2007). Sixty-six male Syrian golden hamsters (Mesocricetus auratus, Botucatu, São Paulo, SP, Brazil), weighing between 80 g to 100 g, were randomly divided into eleven experimental groups: control (C) - 30 minutes of ischemia; 1ischem1′ - 1 minute of ischemia (IP) followed by 20 minutes of waiting time and 30 minutes of ischemia; 3ischem1′ - three 1 minute periods of ischemia with 10-minute intervals between them (IP), followed by 20 minutes of waiting time and 30 minutes of ischemia; 5ischem1′ - five 1 minute periods of ischemia with 10-minute intervals between them (IP), followed by 20 minutes of waiting time and 30 minutes of ischemia; 1ischem5′ – 5 minutes of ischemia (IP) followed by 20 minutes of waiting time and 30 minutes of ischemia; 3ischem5′ - three 5- minute periods of ischemia with 10-minute intervals between them (IP), followed by 20 minutes of waiting time and 30 minutes of ischemia; 3ischem1′_Hista – three one minute periods of ischemia with 10-minute intervals between them (IP), followed by 20 minutes of waiting time and the topical application of histamine (2 µM); 3ischem5′_Hista - three 5 minutes periods of ischemia with 10-minute intervals between them (IP), followed by 20 minutes of waiting time and topical application of histamine (2 µM); control_Hista - topical application of histamine (2 µM); BK - preconditioning with bradykinin (BK) was achieved by the topical application of 400 nM BK for 5 minutes, followed after 20 minutes by either 30 minutes of ischemia or no treatment (control) (Figure 1).
The experimental protocol of the present study. Groups with one, three, and five one-minute ischemic periods: 1ischem1′, 3ischem1′, and 5ischem1′. Groups with one and three five-minute ischemic periods: 1ischem5′ and 3ischem5′. Groups with three one-minute and three five-minute ischemic periods followed by histamine: 3ischem1′-Hista and 5ischem1′_Hista. All ischemic periods had 10-minute rest intervals between them. Bradykinin group (BK) and three control groups: ischemia/reperfusion, histamine, and bradykinin (control, Control_Hista, Control_BK).
The hamster cheek pouch was prepared according to studies by Duling (1973) (20), Svensjö et al. (1978) (21), modified by Persson et al. (1985) (12) and by Bouskela and Grampp (1992) (22) (Figure 2A and B). Anesthesia was induced by an intraperitoneal injection of sodium pentobarbital (0.2 ml/100 g body weight, pentobarbital sodium, Sanofi, Paris, France, 60 mg/ml). After cannulation of the femoral vein, anesthesia was maintained with an injection of alpha-chloralose (2.5 ml/kg body weight, Merck, Darmstadt, Germany). Thirty minutes after the preparation was completed, fluorescein-labeled dextran (FITC-dextran, MW = 150 kDa; 250 mg/kg body weight) was injected intravenously as a macromolecular tracer.
The cheek pouch (Figure 2B) was subjected to ischemic periods of different durations with an inflatable cuff made of thin latex tubing, mounted around the neck of the everted pouch where it leaves the mouth of the hamster. The cuff did not disturb the local blood flow, and an intracuff pressure of 200–220 mmHg created by air compression with a syringe resulted in complete arrest of the microvascular blood flow within few seconds (12).
Increases in microvascular permeability for large molecules (plasma leakage) was quantified by counting sites with extravasation of fluorescent plasma (leaky sites = leaks) at post-capillary venules at 2–5 min intervals after the onset of reperfusion and/or topical application of bradykinin (400 nM) or histamine (2 µM) for 5 minutes. The results are reported as the number of leaks counted 10 minutes after the onset of reperfusion.
Statistical AnalysisResults are reported as means±standard deviations unless otherwise noted. For statistical analyses, ANOVA was used to compare groups to controls. p<0.05 was considered to indicate a statistically significant difference.
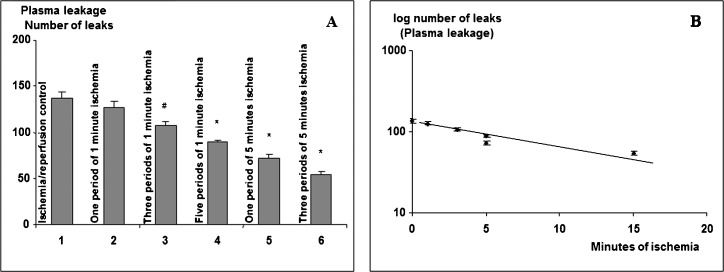
RESULTSBrief 1- to 5-minute periods of ischemia followed by reperfusion caused no visible increases in the number of leaks or plasma leakage. One period of 1 minute of ischemia had influence (p>0.05) on the subsequent 30-minute period of I/R on plasma leakage, but several brief ischemic periods reduced the response to the 30-minute period of ischemia as shown in Figure 3A. Three periods of 5 minutes of ischemia produced a significant reduction of the response to the subsequent 30 minutes of I/R. When logarithmic values of the maximal number of leaks induced by 30 minutes of ischemia were plotted versus the total duration of ischemia during IPC, a linear correlation could be observed (Figure 3B).
Effect of brief periods of ischemia and reperfusion prior to a period of 30 minutes of ischemia. A. Plasma leakage (maximal number of leaks±SD) induced at reperfusion after 30 minutes of ischemia following brief periods of ischemia/reperfusion in six groups of hamsters (each group, n = 6) prior to the final 30 minute-ischemic period. # = p< 0.05, ∗ = p<0.01 as compared with I/R controls. B. Log-linear relationship between the number of leaks and the total ischemic period prior to the final 30 min I/R.
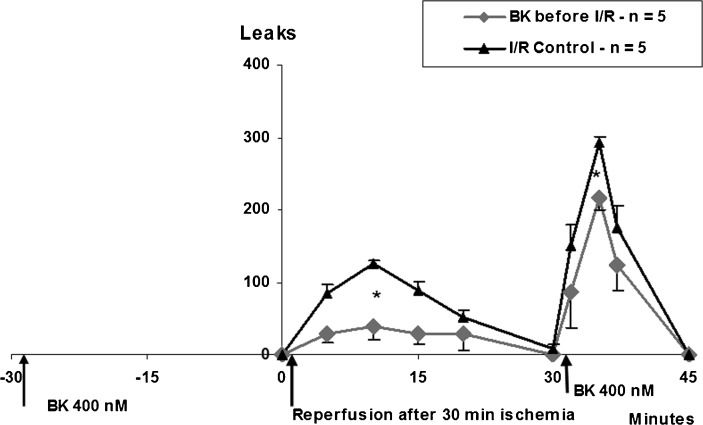
Figure 4 shows two IPC groups with three one-minute and three five-minute ischemia periods followed by the topical application of histamine compared to control. Three occlusions of five minutes produced the fewest leaks compared to the control group. Bradykinin also contributed microcirculatory protection. Five-minute topical applications of 400 nM bradykinin given 30 minutes prior to I/R reduced the response to I/R-induced plasma leakage from 125 to 40 leaks/cm2 (32% of the I/R control group) and reduced the bradykinin-induced plasma leakage from 305 to 226 leaks/cm2 (74% of the control group) (Figure 5).
Effect of brief periods of I/R prior to histamine application. Plasma leakage (maximal number of leaks±SD) induced by histamine (2 µM). Three periods of 1 or 5 minutes of ischemia reduced the subsequent response to histamine. ∗ = p<0.05, # p<0.01 as compared with histamine controls (2 µM).
Plasma leakage (maximal number of leaks±SD) induced by ischemia/reperfusion (I/R) and 400 nM bradykinin (BK) without (Triangles) or with (Diamonds) bradykinin prior to I/R. Bradykinin prior to I/R reduced the subsequent response to I/R (from 125 to 40 leaks - 32% of control) and to bradykinin (from 305 to 225.7 leaks - 74% of control), ∗ p<0.05.
Table 1 displays the results as means and standard deviations of leaks in the 3ischem1′, 3ischem1′_Hista, 3ischem5′, and 3ischem5′_Hista groups. Additionally, it is possible to visualize the percentage of leaks from each group with the associated control values. There was no statistical difference between the 3ischem1′ and 3ischem1′_Hista groups concerning the percentage of leaks. However, the decreases in the percentage of leaks from the 3ischem5′ and 3ischem5′_Hista groups compared to 3ischem1′ and 3ischem1′_Hista were highly significant. Further, the decrease in the percentage of leaks was much more evident in the 3ischem5′ group than in the 3ischem5′_Hista group.
Comparison of the effects of three ischemic periods (of one minute or three minutes duration) on subsequent I/R (30min) and on histamine (2 µM) induced plasma leakage.
| I/R Control | I/R after 3 ischemic periods of one minute | I/R after 3 ischemic periods of five minutes | Control histamine | Histamine after 3 ischemic periods of one minute | Histamine after 3 ischemic periods of five minutes | |
|---|---|---|---|---|---|---|
| Number of leaks± SD | 137±7 | 107±4 | 54±3 | 386±27 | 306±15 | 245±11 |
| % | 100 | 78 | 39 | 100 | 79 | 63 |
| p-value | - | <0.05 | <0.01 | - | <0.05 | <0.01 |
The hamster cheek pouch as prepared for intravital microscopy has been used in several studies to elucidate the mechanisms behind I/R-induced increases in plasma leakage. A 30-minute period of ischemia was originally selected because it resulted in statistically significant and reproducible increases in plasma leakage during reperfusion, whereas 5-minute ischemic periods produce no visible changes in plasma leakage (12). However, the possible effect of IPC on I/R-, histamine and bradykinin induced plasma leakage has not been studied in the HCP.
This study confirmed that repeated minor episodes of ischemia have a protective effect on the microcirculation. This is a natural phenomenon where several primary pathways converge to cardioprotective effect (24,25). Not all of the mechanisms involved in this cardioprotection are known, and our first question in this study was how long the cheek pouch vessels should be occluded to decrease the microvascular permeability during I/R. We found that a one-minute ischemic period was not enough to reduce the number of leaks during reperfusion. We tested three and five one-minute ischemic periods with ten-minute reflow periods, and the results revealed a decreasing number of leaks. The greatest reduction in plasma leakage induced by 30 min I/R were observed with one and three five-minute ischemic periods with the same reflow time. Murry et al. used 15-minute occlusions to precondition myocardial tissue in dogs with high mortality (5). When they attempted to use more occlusions with smaller durations, including four five-minute occlusions with 5-minute reflow between them, they observed protective effects on the myocardium. At 10 minutes reflow period, the maximum number of leaks occured and for that reason we have chosen this time to report. We could also demonstrate that the log of IPC-induced reduction was linearly correlated to the total duration of ischemia for 15 minutes (Figure 3B) suggesting that the preconditioning effect was related to the total time of ischemic rather than the number of ischemic periods.
Preconditioning can protect the heart and other tissues from a subsequent sustained period of ischemia. Early preconditioning is independent of protein synthesis, and the prolonged insult should be less than 2 hours (28). Our model used 30 minutes of total ischemia. Prolonged ischemia will produce impaired endothelial-dependent vasodilation, capillary plugging, increased adhered and transmigrated leukocytes and venous protein leakage (28). Ischemic preconditioning protects endothelial cells and reverses microcirculatory injury caused by ischemia (3).
The effect of IPC (3×1 min) on histamine-induced plasma leakage did not differ from that observed with I/R; the respective effects were 79% and 78% of the non-IPC-control group response. When the IPC-period was prolonged (3×5 min), further 63% and 40% reductions were observed in the histamine and I/R groups, respectively, compared to the non-IPC-control group. A similar histamine-preconditioning effect was observed by Erlansson et al. (1985), who reported that in a series of four repeated histamine challenges, the second, third, and fourth challenges produced plasma leakages that were each 65% of the first challenge (12). Taken together, these results may suggest that the preconditioning effect is more related to the strength of the stimuli (the duration of ischemic periods or the dose of histamine) on endothelial cells, rather than the nature of preconditioning (IPC or histamine). In this context, it is interesting to note that in a study using HCP an antioxidant (flavonoid) reduced the plasma leakage responses induced by histamine, bradykinin and I/R to 26%, 32% and 31%, respectively, of the control group without antioxidant pretreatment (23).
Delbin et al. have shown that histamine did not influence pulmonary ischemia/reperfusion injury in sedentary or preconditioned exercised rats (five days a week for eight weeks) (29). However, increased nitric oxide (NO) release after regular physical exercise is cardioprotective (30). Perhaps ischemic preconditioning protection is different from the protection developed from exercise training. Indeed, IPC prevented the impairment of pulmonary endothelium-dependent vasodilation partially due to nitric oxide (NO) release in isolated rat lungs (31).
In this study we observed a significant preconditioning effect of three one-minute and three five-minute ischemic periods (Table 1) when tested with 30 min I/R and with histamine (2µM). There was no difference in the preconditioning effect with one-minute ischemic periods (78 % and 79% of control). However, the three five-minute ischemic periods induced a greater preconditioning effect in the 30 min I/R group (39% of control) than in the histamine group (63% of control) suggesting a difference in the mechanism of plasma leakage induction due to ROS formation and the direct receptor stimulation with histamine. Indeed during ischemia in the HCP the presence of reactive oxygen species have been observed (32), and with the addition of histamine, calcium channels may exhibit more prominent action after three five-minute ischemic periods. Some studies have shown the contribution of histamine to ischemic preconditioning and microcirculation. Kandilci et al. prevented the decrease in pulmonary vasodilator response to histamine and acetylcholine following two hours of hypothermic ischemia in rats using two 5-minute cycles of ischemia and reperfusion (33). Moreover, Wang et al. demonstrated that mast cell granules are not affected by ischemic preconditioning. The theory behind this finding was that an initial brief ischemia would result in mast cell degranulation without significant cardiovascular damage, but when a prolonged ischemic episode would occur, no more degranulation could be possible (34).
We have observed the preconditioning effect of bradykinin on ischemia/reperfusion by reducing plasma leakage compared to the control group. In clinical practice, exogenous administration of bradykinin prior to cardiopulmonary bypass in coronary artery bypass grafting reduces myocardial injury (35). Bradykinin can also be considered an endothelium protector. Indeed, in vitro experiments with pig cerebral endothelial cells exposed to necrotic and apoptotic cell inducers (H2O2) demonstrated enhanced expression of the cytoprotective proteins COX-2 and (Cu/Zn) SOD when the cells were pretreated with bradykinin (36). Nevertheless, nitrite production in response to bradykinin was enhanced in microvessels during second window protection (SWOP) in dogs subjected to myocardial preconditioning with 10 minutes of coronary artery occlusion (37). Further, endothelial nitric oxide synthase (eNOS) protein in the SWOP myocardium was twofold higher than in control groups.
Our study has demonstrated a preconditioning effect of short periods of ischemia and reperfusion when measured as plasma leakage induced by a 30 min I/R-period. This preconditioning effect (reduction in the response to 30 min I/R-induced plasma leakage) that was log-linear related to the total time of brief ischemic periods. When the same preconditioning protocol was applied before histamine application a similar but smaller reduction in the plasma leakage was observed. A preconditioning effect was also observed after topical application of bradykinin 30 minutes prior to I/R-induced plasma leakage that was of the same magnitude as that induced by brief ischemic periods.
No potential conflict of interest was reported.