Primary cutaneous melanoma still constitutes the main cause of skin cancer death in developed countries, and its incidence in recent years has been increasing in a steady, worrisome manner.
OBJECTIVES:This study evaluated the clinical, epidemiological and demographic aspects of this disease, and correlated them with patient prognosis.
METHODS:Using epidemiologic and clinical data, we analyzed 84 patients with mild to severe primary cutaneous melanoma treated between 1990 and 2007. Slides containing surgical specimens were analyzed, and new slides were made from archived paraffin sections when necessary.
RESULTS:The melanoma incidence was higher in areas of sun exposure, with lesions commonly observed in the trunk for males, and lower limbs for females. In addition to Breslow’s thickness and ulceration (p = 0.043 and p < 0.001, respectively), the mitotic rate per mm2 also correlated with worse patient outcome (p = 0.0007). The sum of ulceration (0 when absent or 1 when present), the Breslow index (1 when <1 mm, 2 when >1 mm and <4 mm, 3 when >4 mm) and the mitotic index (0 when absent or 1 when ≥1 per mm2) allowed the establishment of a prognostic score: if the sum was equal to or over three, nearly all (91.7%) patients had systemic disease. The 5-year survival was approximately seventy percent.
CONCLUSION:Because American Join Committee of Cancer Staging will update the classification of malignant tumors (TNM) staging in the near future, and introduce mitosis as a prognostic factor, our results show the importance of such a feature. Additional studies are necessary to confirm the importance of a prognostic score as proposed herein.
Melanoma is a tumor of neuroectodermal origin that is formed from melanocytes - cells that are found along the basal layer of the epidermis with the main function of producing the pigment melanin.1 It has been showing a steady, worrisome increase in incidence that is likely due to the intermittent habit of sun exposure, which is considered to be the major etiologic factor for skin cancer.2
Melanoma is a neoplasm with good prognosis when diagnosed early. Nevertheless for intermediate thickness (1 to 4 mm in depth), it is not possible to predict the disease progression, varying from 63 to 89% for 5-year survival. However, when deeper than 4 mm, the 5-year survival rates are low (33 to 55%).3–5 The transition from radial growth to vertical growth grants melanoma with higher aggressiveness, the larger and deeper the lesion is.1
Melanoma shows great potential for dissemination, which is the reason it constitutes one of the most severe tumors among skin lesions, with high mortality rates when diagnosed late.6–8
However, even with large tumor resections,9 the 5-year survival rates remain low, resulting in the search for new treatment methods. Thus, several studies conducted in the 1950s and 1960s sought to establish melanoma-related predictive factors.
In 1969, Clark et al.10 proposed staging criteria for lesions on the basis of skin invasion levels. Subsequently, Breslow and Macht 11 evidenced the importance of the primary melanoma thickness in millimeters, and this index became the most important prognostic indicator,12, 13 in association with data on ulceration, mitosis and regression.5,14,15
Preventive campaigns, dermatoscopy, therapeutic approach protocols, determination of anatomic-pathologic prognostic factors, and more accurate staging through the study of sentinel lymph nodes have allowed for the early diagnosis, and treatment of melanomas in the last decade.16
Although variables such as clinical presentation, histopathological data and staging are considered to be appropriate instruments for evaluating the prognosis of melanoma cases, these variables are not always sufficient to accurately predict the evolution of a specific case.14 Patients with similar clinical and anatomic-pathologic prognostic factors may progress in different manners, sometimes progressing favorably, sometimes with disease dissemination, probably because of the intrinsic properties of these tumor cells. A number of studies have examined molecular factors that may explain such differences, particularly focusing on genetic changes involved in tumorigenesis and melanoma progression.2, 17– 19
OBJECTIVES- •
To study the clinical and epidemiologic aspects in primary skin melanomas.
- •
To survey histopathological data (Breslow index, Clark level, ulceration, mitotic index and regression) revised according to the Brazilian Melanoma Group’s protocol.
- •
To correlate these data with primary cutaneous melanoma-treated patient evolution.
This retrospective study comprises 84 primary cutaneous melanoma-diagnosed patients who underwent surgical treatment between 1990 and 2007, in private practice (50 cases) and at the Sistema Unico de Saude (SUS, Unified Health System) unit (34 cases).
Clinical, epidemiologic, histological and disease evolution data were collected at the pre- and post-operative follow-up of study patients. The histological preparations were reviewed, and new slides were obtained from paraffin blocks when necessary, and stained with hematoxylin and eosin (HE), with the prognostic parameters being evaluated in compliance with the protocol set by the Brazilian Melanoma Group.20
Demographic data (age, sex and ethnicity), topographic location and histopathological data (Breslow index, Clark level, ulceration, mitotic index, regression and peri- and intra-tumor infiltration) were surveyed for the treated cases.
Variables were described in frequencies or means and standard deviations, and they were compared with the Chi-square test, Fisher exact test and Student’s t test.
The evaluation of disease-free survival and cancer-specific survival was performed through actuarial analysis, and confirmed using the Kaplan-Meier Product.21 In this analysis, only melanomas ≥ 1 mm were included.
For result analysis, the rejection probability of the 95% hypothesis (p ≤ 0.05) was adopted.
The study was approved by the Research Ethics Committees of the University of Sao Paulo Medical School (16/02/2005), A C Camargo Hospital (15/10/2007), and Brigadeiro Hospital (05/09/2007).
RESULTSThere were no significant differences of frequency between genders (Table 1), (p = 0.1266). Only 1 African-American patient participated in the study (1%); the remaining patients were Caucasian.
Clinical and epidemiologic findings in 84 patients with primary cutaneous melanoma
| Variables | Males | Females | Overall Group |
|---|---|---|---|
| 41.7% | 58.3% | ||
| Age (mean ± standard deviation) | 56.0±17.9 years | 53.9±18.4 years | 54.8±18.1 years |
| Decades of life | |||
| - up to the 3rd | 11.4% | 12.2% | 11.9% |
| - 4th | 5.7% | 16.3% | 11.9% |
| - 5th | 14.3% | 24.5% | 20.2% |
| - 6th | 20.0% | 6.2% | 11.9% |
| - 7th | 31.5% | 16.3% | 22.6% |
| - 8th | 11.4% | 16.3% | 14.3% |
| - 9th | 5.7% | 8.2% | 7.2% |
| Lesion site | |||
| - Head and neck | 31.5% | 18.4% | 23.2% |
| - Lower limbs | 14.3% | 36.7% | 27.6% |
| - Upper limbs | 11.4% | 24.5% | 21.8% |
| - Trunk | 42.8% | 20.4% | 27.4% |
| Breslow index | |||
| - in situ | 11.4% | 18.4% | 15.5% |
| - < 1 mm | 34.3% | 42.8% | 39.3% |
| - 1–2 mm | 17.1% | 16.3% | 16.7% |
| - 2–4 mm | 25.8% | 10.3% | 16.7% |
| - > 4 mm | 11.4% | 12.2% | 11.8% |
| Clark levels | |||
| - I | 14.3% | 18.4% | 16.7% |
| - II | 14.3% | 12.2% | 13.1% |
| - III | 40.0% | 36.7% | 38.0% |
| - IV | 31.4% | 24.5% | 27.4% |
| - V | 0 | 8.2% | 4.8% |
This patient group’s mean age was 54.8±18.1 years, which was similar for both genders: 56.0±17.9 for males and 53.9±18.4 for females (p = 0.6190), and ranged from 13–91 years old. In addition, there were no significant age differences between genders when they were compared by decade of life (p = 0.1942).
Melanomas are predominant in the trunk in males (42.8%), and the lower limbs in females (36.7%) (p = 0.0113).
There was no evidence of differences between genders when the mean ages and standard deviations were assessed for the location of lesions.
Comparing location of the lesions in relation of the patient gender there was a significant difference (p = 0.0113), being lesions in the head and neck and in the trunk particularly more frequent in males, while lesions in the upper and lower limbs are more frequent in females (Figure 1).
When analyzing the curves representing mean ages in relation to lesion sites in the male group, the following was observed: head and neck lesions appeared at significantly older ages than lower limb (p < 0.001) and trunk (p < 0.001) lesions. Similarly, upper limb lesions affected older males significantly more than lower limb (p = 0.014) and trunk (p = 0.007) lesions.
In the female group, head and neck lesions affected older patients significantly more than lower limb (p = 0.020) and trunk (p = 0.004) lesions; in the remaining locations, there were no significant differences.
Among the morphological parameters, there was homogeneous distribution of patients regarding depth (Breslow) of lesions, although there was a higher frequency of lesions smaller than 1 mm (54.8%). There was uniform distribution regarding Clark levels (p = 0.461).
There was no evidence of differences in the tumor Breslow index, in terms of mean age (and standard deviations); however, there was a wide age range in these groups. In addition, no significant differences were observed when Clark levels were analyzed with respect to patient age.
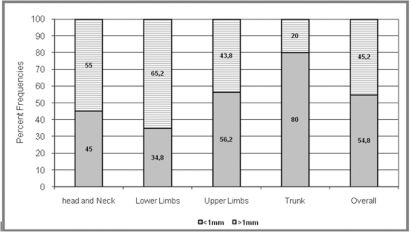
Breslow index frequencies, as distributed according to lesion sites, were widely varied. In Figure 2, we observed that there was a higher frequency of tumors thicker than 1 mm (65.2%) in the lower limb lesions, while tumors less than 1 mm thick were significantly predominant in trunk lesions (p < 0.001).
The Clark level distribution according to tumor location demonstrated that level III tumors were predominant in the lower and upper limbs and the trunk (p < 0.001), while there was no difference in the distribution in the head and neck.
To ensure a minimum 24 months follow-up in patient disease-free and overall survival evaluation, melanoma diagnosed and treated as of October 2006 were excluded, as well as in situ cutaneous melanoma, and predisposing disease (xeroderma pigmentosum).
Of the 54 patients (27 with melanoma ≥ 1 mm and 27 with melanoma < 1 mm) included in this evaluation, disease dissemination was observed in 12 patients (22.2%), of which 10 (18.5%) progressed to death, while 2 were still on oncological follow-up (3.7%). Disease dissemination was not observed in any of the patients with < 1 mm-thick melanoma.
There was no difference of disease dissemination between genders (p = 0.577), and was observed in 50% of females and 53.3% of males. The mean age of patients with disseminated disease (60.7±21.1) was not different (p = 0.850) from that of patients without relapse (59.3±15.2). In addition, there was no difference in the distribution of frequencies relative to the melanoma site (p = 0.950).
The differences found in the patient evolution analysis in relation to the Breslow indexes (Table 2) were statistically significant (p = 0.042), revealing that 50% of patients with disseminated disease had > 4 mm-thick melanomas as compared to 13.3% of patients with no disease dissemination. This difference was significant (p = 0.043) when Breslow index means were compared between disseminated (4.6±2.8 mm) and non-disseminated (2.6±2.3 mm) melanomas.
Ulceration was also significantly associated with unfavorable patient evolution, affecting nine out of twelve cases with melanoma dissemination (p < 0.001), as seen in Table 3.
Distribution of frequencies relative to the evolution of 54 patients with melanoma according to the presence or absence of ulceration
| Ulceration | Disseminated disease | Total | ||||
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| N | % | N | % | n | % | |
| Yes | 3 | 7.1 | 9 | 75.0 | 12 | 22.2 |
| No | 39 | 92.9 | 3 | 25.0 | 42 | 77.8 |
| Total | 42 | 100.0 | 12 | 100.0 | 54 | 100.0 |
Freedom degree: 1. Calculated chi-square: 28.94. Calculated p value: < 0.001
The mitotic index was correlated to Breslow index (p = 0.0002), as seen in Table 4. The number of mitoses was significantly higher in patients with disease progression (p = 0.0171), as shown in Table 5.
Distribution of frequencies relative to the number of mitoses per mm2 according to Breslow’s index
| Breslow index | Number of mitoses per mm2 | Total | ||||
|---|---|---|---|---|---|---|
| Zero | ≥ 1 | |||||
| n | % | n | % | n | % | |
| <1 mm | 18 | 81.8 | 9 | 28.1 | 27 | 50.0 |
| ≥1 mm | 4 | 18.2 | 23 | 71.9 | 27 | 50.0 |
| Total | 22 | 100.0 | 32 | 100.0 | 54 | 100.0 |
Fisher exact test. Calculated p value: 0.0002.
Distribution of frequencies relative to the evolution of 54 patients with melanoma according to the number of mitoses per mm2
| Number of mitoses per mm2 | Disseminated disease | Total | ||||
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| n | % | n | % | n | % | |
| Zero | 21 | 50.0 | 1 | 8.3 | 22 | 40.7 |
| ≥1 | 21 | 50.0 | 11 | 91.7 | 32 | 59.3 |
| Total | 42 | 100.0 | 12 | 100.0 | 54 | 100.0 |
Fisher exact test. Calculated p value: 0.0171.
A sentinel lymph node biopsy was performed in 19/54 patients, of which 2 had lymph nodes metastasis. One case evolved to dissemination and death, while the other is still on clinical follow-up.
Among patients with melanoma dissemination, the mean disease-free survival time was 17.8 ±18.3 months (range, 4–65 months). The actuarial analysis, as confirmed using the Kaplan-Meier Product, revealed an 82% disease-free rate for 1 year, 70% for 2 years and 62% for 5 years.
Cancer-specific mean survival time was 24.5 ±20.7 months (range, 5–67 months). Survival rates, as calculated by the actuarial analysis and Kaplan-Meier Product, revealed an 85% survival rate for 1 year, 82% for 2 years and 70% for 5 years.
DISCUSSIONMalignant cutaneous melanoma is still a major clinical challenge, especially when we take into consideration the increasing incidence of smaller lesions that have been recorded in the last decades in developed countries,22 and more notably, in their Caucasian populations.3 Melanoma is still the main cause of death due to skin cancer.14
In the south region of Brazil, the relative increase of melanoma incidence between 1979 and 1987 was 38% among men and 11% among women.3 Such an increase, although in lower proportions, was subsequently confirmed in surveys carried out in Sao Paulo between 1963 and 1997, 23 and between 1993 and 2006,24 and in Goiania from 1988 to 2000.25
These Brazilian data are not different from the results of epidemiologic studies conducted in other countries, like Scotland (a rise of 303% for men and 187% for women over eleven years),13 the United States (increasing incidence of cutaneous melanoma),26,27 Queensland, Australia (increasing by more than 50% in women and more than doubling in men), 28 Sweden (5% annual increase),29 New South Wales, Australia (incidence rates are much higher than a few decades before),30 Japan (annual increase ratio for deaths of 6.3%)31 and British Columbia, Canada (3–7% a year frequency increase for new cases, even doubling at each 10–15 year period, depending on the population studied)32.
The available diagnostic methods for early detection of the disease, based on microscopic findings of the tissue morphology, still lack accuracy.1 Likewise, the clinical and histological variables determining the disease prognosis include the Breslow index, tumor size, presence of ulceration, mitotic index and level of vascular invasion, but gaps in the definition of risk groups persist, as this neoplasm’s clinical manifestation is quite variable from case to case.14, 33
From an epidemiological perspective, we noticed that this pathology affected men and women equally often and at similar mean ages, corroborating the data found in the international and national literature.4,22,23,34 Reports of differences between genders in the incidence of cutaneous melanoma appear to be related to each country’s culture, in terms of which part of the body is exposed to the sun more often and by whom (Jordan).35
Among males trunk lesions were more frequent (42.8%), while among females the lower limb lesions prevaled (36.6%). Such results confirm the relation of cutaneous melanoma with solar radiation, as the higher melanoma incidence in areas that were more exposed to the sun resulted in a disease incidence in different locations in males and females, according to their habits and vestments in Brazil. These findings are consistent with reports identifying the most frequent tumor sites as the backs of males (49.5%), and the lower limbs of females (33.1%) in the southern region of Brazil.36 Criado et al.23 had even observed a likelihood 2.26-times as high for males to develop cutaneous melanoma in the posterior region of the trunk, while the likelihood for the disease to occur on the lower limbs was 2.4-times as high in females.
There was no evidence of differences between genders when assessing mean age, Clark level and Breslow index, except for lesions having a thickness of 2 to 4 mm, which were significantly more frequent in males (p = 0.049).
The increased incidence currently being reported for this neoplasm generally refers to thinner or in situ lesions.13,29 However in males, an increase in the number of patients with thicker lesions has also been demonstrated.16,27
This study identified an 18.5% rate of disease dissemination resulting in death, and a 3.7% rate of disease dissemination without death. Therefore, 22.2% of patients had disease dissemination, with no differences between males and females and no influence by patient age or lesion site.
Disease dissemination was observed an average of 17.8 months from lesion diagnosis, and deaths occurred an average of 24.5 months from the initial diagnosis. There was no correlation between these mean times and lesion thickness, suggesting an association of ≥ 1 mm-thick lesion dissemination risk with other characteristics.
The Breslow index constituted a predictive variable of disease evolution, as no patient with a < 1 mm-thick tumor progressed to disease dissemination, while patients with ≥ 1 mm-thick tumors had dissemination, particularly those with > 4 mm-thick tumors, confirming the reports of other publications.4,9,15
Likewise, the number of detected mitoses in the primary tumor may follow the progression of the disease (r = 0.43), and it may constitute, along with ulceration and Breslow index, the group of associated factors that can best predict patient tumor evolution.
Of the 27 ≥ 1 mm melanoma cases used for disease-free and survival time analysis, 12 had ulcerated lesions, and 9 of those 12 lesions had disease dissemination. Of the patients with a Breslow index greater than 4 mm, 7 had ulceration, and 6 of those 7 ulcerations progressed to death or dissemination.
Breslow index association with the presence of ulceration and mitotic index showed a clear correlation with patient evolution when converted into a numeric score: sum of ulceration (0 when absent or 1 when present), Breslow index (1 when < 1 mm, 2 when ≥ 1 and < 4 mm, 3 when ≥ 4 mm) and mitotic index (0 when absent or 1 when ≥1 per mm2).
The study also identified a 63% disease-free survival time rate, and a 70% cancer-specific 5-year survival rate. In São Paulo, Brazil, Criado et al.22 reported a similar 5-year survival (67.4%) to that found in Great Britain by Carmichael et al.32 (55.5% for males and 70.3% for females).
Distribution of frequencies relative to the disease evolution of 54 patients with melanoma in relation to the sum of the Breslow index, ulceration and the mitotic index
| Indicator* (Score) | Disseminated disease | Total | ||||
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| n | % | n | % | n | % | |
| 5 | 1 | 2.4 | 6 | 50.0 | 7 | 13.0 |
| 4 | 2 | 4.8 | 4 | 33.3 | 6 | 11.1 |
| 3 | 9 | 21.4 | 1 | 33.3 | 10 | 18.5 |
| 2 | 11 | 26.2 | 1 | 8.3 | 12 | 22.2 |
| 1 | 19 | 45.2 | 0 | 0.0 | 19 | 35.2 |
| Total | 42 | 100.0 | 12 | 100.0 | 54 | 100.0 |
- •
Melanoma is a low-incidence skin tumor that is showing an increasing incidence in the last decades.
- •
A higher incidence of melanoma was found in sun-exposed areas, with trunk lesions being more frequent in males, and lower limb lesions being predominant in females.
- •
The mean time elapsed from treatment to melanoma dissemination diagnosis was 17.8 months.
- •
The survival rates calculated through actuarial analysis and the Kaplan-Meier Product revealed a 5-year survival rate of seventy percent.
- •
Deeper tumors correlated with more unfavorable patient disease evolution, which correlated with the Breslow index.
- •
The presence of ulcer and mitotic index had also impact in patient disease evolution.