The influenza A (H1N1) resurgence was identified in April of 2009 in North America, 35 years after its initial description.1 Since then, cases of influenza A (H1N1) have been reported on all continents, with the clinical presentation ranging from mild symptoms (runny nose, fever, cough, and myalgia) to acute respiratory distress syndrome (ARDS).2-4 Approximately 75% of patients with influenza A (H1N1) admitted to an intensive care unit (ICU) required invasive mechanical ventilation,5 one-third of whom progressed to refractory hypoxemia and needed rescue ventilation techniques, including alveolar recruitment maneuvers, ventilation in the prone position, high-frequency ventilation, extracorporeal membrane oxygenation, or inhaled nitric oxide.6-7 In addition to the ventilatory support, treatment for respiratory failure due to influenza A (H1N1) includes antiviral agents, which should be initiated at the time of clinical suspicion, preferably within 48 hours of the onset of symptoms. We describe herein the case of a patient with ARDS secondary to influenza A (H1N1) on whom recruitment maneuvers and ventilation in the prone position were used for the treatment of refractory hypoxemia, along with corticosteroids, oseltamivir, and intravenous zanamivir.
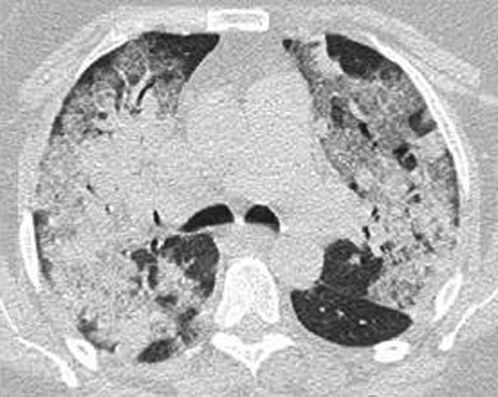
CASE REPORTA 63-year-old woman from Campinas, SP, Brazil was admitted to the ICU on August 21, 2009 (Day 1) because of respiratory failure. She had a five-day history of dry cough, myalgia, wheezing, and fever (38°C) and had been using clarithromycin for three days. Her past medical history was significant for hypertension, type 2 diabetes mellitus, obesity (body mass index of 30.1 kg/m2), and total hip replacement 10 months before admission complicated by deep-vein thrombosis (DVT) and pulmonary embolism, both of which were successfully treated. Upon admission, she was placed in respiratory isolation with negative pressure, and nasopharyngeal washings were collected for detection of influenza A (H1N1) by reverse-transcriptase polymerase chain reaction (RT-PCR). The blood and tracheal aspirate cultures were negative, as was the urinary assessment for Pneumococcus and Legionella. Arterial blood gas analysis confirmed severe hypoxemia, and an X-ray computed tomography (CT) scan (Figure 1) of the thorax showed bilateral pulmonary infiltrates.
Orotracheal intubation was performed after a failed attempt at noninvasive ventilation. Oseltamivir (150 mg b.i.d.) administered through the enteral route and intravenous ceftriaxone (1 g IV b.i.d.), levofloxacin (500 mg IV q.d.), vancomycin (1 g IV b.i.d.) and methylprednisolone (2 mg/kg/day) were started. A Doppler ultrasound of the lower limbs was negative for DVT, and an echocardiogram showed a systolic pulmonary artery pressure of 26 mm Hg with no signs of right ventricular dysfunction and a left ventricular ejection fraction of 66%.
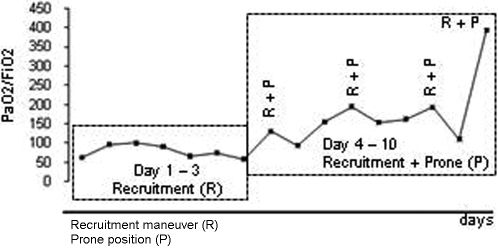
Severe hypoxemia (PaO2 55 mm Hg) was present despite ventilation with a positive end-expiratory pressure (PEEP) of 16 cm H2O and pure oxygen, so a recruitment maneuver was performed for two minutes using a PEEP of 35 cm H2O and a plateau pressure of 50 cm H2O. After the recruitment, the PEEP was titrated at 18 cm H2O according to the best dynamic compliance, but there was no significant improvement in the PaO2/FiO2 ratio (Figure 2). A decision was then made to repeat the recruitment maneuver with the patient in the prone position, which resulted in significant improvement in gas exchange (Figure 2). Mechanical ventilation in the prone position for an average of 12 hours/day and one daily recruitment maneuver were continued for three consecutive days, with progressive improvement in the gas exchange (Figure 2). At all times, we maintained a protective ventilatory strategy with low tidal volumes (6 mL/kg of ideal body weight) and a plateau pressure of <30 cm H2O.
On the 12th day of her ICU stay, amantadine was added to the treatment regimen, and importation of intravenous zanamivir (not approved in Brazil) was requested because the RT-PCR for influenza A (H1N1) remained positive. On the 19th day of her stay in the ICU, intravenous zanamivir was started, and the RT-PCR for influenza A (H1N1) became negative two days later. Because the patient still had diffuse, patchy ground-glass opacities on chest CT and had signs of incipient interstitial fibrosis, pulse therapy with methylprednisolone (1 g/day) for three consecutive days was given. The patient showed progressive radiological and gas exchange improvement and was released from mechanical ventilation 26 days after intubation. She was discharged from the ICU 30 days after admission and discharged home 3 weeks later.
DISCUSSIONWe reported the successful use of mechanical ventilation in the prone position combined with recruitment maneuvers as rescue therapies for refractory hypoxemia in a patient with ARDS due to influenza A (H1N1). We also described the use of intravenous zanamivir in a patient with persistently positive RT-PCR for influenza A (H1N1).
Because of high suspicion during the southern hemisphere epidemic, even nonspecific symptoms of dry cough, fever, and myalgia triggered the application of our protocol for managing influenza A (H1N1) at our institution. The protocol includes respiratory isolation (negative pressure and use of an N95 mask by health professionals) to prevent in-hospital transmission of the virus, collection of respiratory specimens for RT-PCR, and immediate therapy with oseltamivir. It is noteworthy that the rapid test for antigen in nasopharyngeal secretions was negative for the patient described here. The rapid test results are available in 15 minutes, but they have a low sensitivity (20%–30%), and a negative result should not rule out influenza A (H1N1) or lead to discontinuation of the respiratory isolation procedures. The RT-PCR (sensitivity of 98%), available within six hours, was positive for the oropharyngeal washings and tracheal secretions. Alternatively, viral culture could have been performed, which has a sensitivity of 89% and available results in two to three days.4
Treatment with antiviral drugs should be started early in serious cases, even before diagnostic confirmation, preferably within 48 hours of the onset of symptoms, because the delay in instituting such treatment may contribute to increased disease severity.3 The use of neuraminidase inhibitors (oseltamivir and zanamivir) is preferable to the use of adamantanes (rimantadine and amantadine) because of the increased resistance of the virus to the latter class of drugs;2 the combination of an adamantane with a neuraminidase inhibitor is recommended by the World Health Organization. In the present case, the RT-PCR remained positive after 12 days of enteral oseltamivir at twice the recommend dose, which led us to add amantadine and later replace oseltamivir with intravenous zanamivir.2
The association of recruitment maneuvers with prone positioning was crucial to the management of the hypoxemia, serving as a bridge to the recovery of the lungs. Alveolar damage associated with necrotizing bronchiolitis with extensive hemorrhage has been described as the major histological pattern for patients with H1N1 pneumonia and respiratory failure,8 and we believe that this type of injury can be limited by alveolar recruitment interventions such as RM, prone positioning, and corticosteroid use. Recruitment maneuvers in ARDS, through the application of high inspiratory pressures for short periods of time1, can be used to open collapsed alveoli and allow a more homogeneous distribution of the ventilation, potentially reducing the lung injury induced by mechanical ventilation. Mechanical ventilation in the prone position can be used as a rescue therapy for patients with refractory hypoxemia.7 Prone positioning leads to better oxygenation by facilitating the recruitment of collapsed alveoli in the dorsal regions of the lungs and through the improvement of the ventilation/perfusion matching caused by a shift in pulmonary perfusion to the ventral regions. The prone position requires training of the ICU staff, particularly with regard to the proper and safe positioning of patients, tubes, and catheters during position changes. We successfully combined the use of recruitment maneuvers with prone positioning to optimize gas exchange in our patient, a strategy seldom described in the literature.10 Other therapeutic options would have been inhaled nitric oxide, high-frequency ventilation, and extracorporeal membrane oxygenation.
This case report illustrates the presentation of severe pneumonia caused by influenza A (H1N1) and the ventilatory and pharmacological measures that can be adopted for the treatment of ARDS associated with refractory hypoxemia.