Our purpose was to compare 2 methods of treatment of chronic infection in hip arthroplasties—with or without an antibiotic-loaded cement spacer.
METHODS:In a prospective study, we treated 68 infected hip arthroplasties with discharging sinuses and bone loss, comparing 30 patients treated in 2 stages without the use of a spacer (control group) and 38 patients treated with a vancomycin-loaded spacer (study group). The average follow-up was 4 years (2-8.5 years). One patient died of unrelated causes 4 months after first-stage surgery and was excluded from the study.
RESULTS:The 2-stage surgery without spacer controlled the infection in 66.7% of patients, and the 2-stage surgery using the spacer controlled it in 89.1% (P < 0.05). At last follow-up, the average Harris Hip Score increased from 19.3 to 69.0 in the control group versus 19.7 to 75.2 in the study group (P > 0.05). The average leg length discrepancy was 2.6 cm in the control group and 1.5 cm in the study group (P < 0.05). The patients treated with a spacer had better clinical results (81.5% of patients with good results against 60.0% for the control group).
CONCLUSION:The use of an antibiotic-loaded spacer in the 2-stage treatment of infected hip arthroplasties provides better infection control with good functional results and is superior to treatment in 2 stages without a spacer. Level of Evidence: Therapeutic study, Level I-1.
As revisões em dois tempos continuam sendo os métodos preferidos no tratamento das artroplastias infectadas do quadril. O procedimento em dois estágios apresenta várias desvantagens teóricas, ainda não comprovadas por estudos comparativos.
MATERIAIS E MÉTODOS:Em um estudo prospectivo, tratamos 68 pacientes com artroplastias infectadas de quadril com perdas ósseas e fístulas ativas, comparando 30 casos tratados em dois tempos sem espaçador (grupo controle) e 38 casos tratados em dois tempos com o uso de um espaçador de cimento adicionado a vancomicina (grupo de estudo). Um paciente faleceu após quatro meses da cirurgia e foi excluído do estudo. O seguimento médio foi de quatro anos (2-8,5 anos).
RESULTADOS:A cirurgia em dois tempos sem espaçador controlou a infecção em 66,7% dos casos comparada a 89,1% (p<0,05) nos casos tratados com espaçador. No último seguimento, o Escore de Harris para Quadril passou de 19,3 a 69,0 no grupo controle e de 19,7 para 75,2 no grupo do estudo (p>0,05). A média de discrepância de membros inferiores foi de 2,6cm no grupo controle e de 1,5cm nos grupo do estudo (p<0,05). O grupo tratado com espaçador teve melhores resultados clínicos ao final do estudo (81,5% de bons resultados comparados a 60,0% do grupo tratado sem espaçador).
CONCLUSÃO:O uso de espaçador adicionado a antibióticos no período intermediário do tratamento das artroplastias infectadas do quadril em dois tempos proporciona melhor controle de infecção, com bons resultados funcionais, sendo superior à cirurgia em dois tempos sem espaçador.
The prevalence of deep infection following hip arthroplasty has been significantly reduced, although its levels still give cause for concern.1,2
The treatment of infection is a long process often requiring more than 1 surgery, causing suffering and giving rise to extremely high financial and socials costs. Nevertheless, the prognosis for resolving infectious processes in hip arthroplasties reaches about 80% to 90% with the current surgical techniques.3
The control of chronic infection in arthroplasties requires the removal of the prosthetic components and extensive debridement. After this, there are 3 types of procedures that may be considered:4–6
- 1)
Simple wound closure and maintenance of the patient without an implant, through the Girdlestone surgery;7 this is a safe method as regards infectious control, but functional outcome is poor.8,9
- 2)
Immediate placement of a definitive prosthesis (1-stage revision), which was widely used by European surgeons during the 1980s and 1990s.7,10,11 Currently, this procedure is contraindicated in patients infected with discharging sinuses, poor condition of soft parts, and bone loss requiring allografts.8,12 Because a 1-stage revision is indicated under conditions requiring the placement of cemented components, cementless arthroplasties should not be performed as a single-stage surgery.13
- 3)
A 2-stage or a 3-stage surgery, which are safest and are used worldwide.14–18
However, the traditional 2-stage procedure has several disadvantages compared with the 1-stage surgery. During the intermediate period, patients stay in hospital for at least 3 weeks, which is the period of skeletal traction required to heal the soft tissues and start rehabilitation.19 Between surgeries, the patient has a shortened leg and is impaired regarding rehabilitation and function. The patient’s loss of mobility increases the risk of pressure ulcers, osteoporosis, and thromboembolism.20 The second stage is more difficult than an aseptic revision due to cicatricial retraction and muscular contractures that occur with leg shortening; bone landmarks are difficult to identify during surgery.19 Disuse osteoporosis impairs the mechanical conditions for the fixation of the permanent prosthesis and predisposes to fractures.21
At the beginning of the 1990s, 2 authors independently described the use of a cement block impregnated with antibiotics to fill large cavities in the acetabular and femoral regions of patients with an infected hip arthroplasty and severe bone loss.22,23 The spacer includes hip prosthesis components coated with PMMA (polymethylmethacrylate) and may be articulated as a total prosthesis24,25 or unipolar as a partial prosthesis.26,27
Regardless of the worldwide acceptance of the spacer as the main method of treatment of infected hip replacements, no objective data have been reported in the literature to prove the real benefits of using a spacer in 2-stage surgeries.2,28
The purpose of this study was to prospectively compare 2 methods for treating chronic infection in hip arthroplasties: 2-stage surgery with and without using an antibiotic-loaded cement spacer.
MATERIALS AND METHODSA prospective study was performed in 68 patients diagnosed with chronic infected hip arthroplasties and treated in our Institution from April 1996 to January 2003.
As inclusion criteria, all of the following were required:
- 1.
Previous surgery with total or partial hip prosthesis;
- 2.
Diagnosis of infection, based on bacterial identification in cultures of samples collected during the first surgery of the treatment;
- 3.
Minimum time period between the arthroplasty and the infectious condition of 4 weeks;
- 4.
Presence of a discharging sinus communicating with the prosthesis;
- 5.
A written informed consent for the performance of the study procedures, where the patient states his/her awareness of the experimental character of the investigation and the possible complications secondary to the treatments.
Sixty-eight patients were randomly selected to receive treatment in 2 stages without a spacer (control group) or with the placement of an antibiotic-loaded cement spacer (study group).
The mean age of patients was 54.6 years (range 16-84 years), with no difference between the groups. The male sex prevailed, with 57.8% of the total number of patients. Etiologies requiring most of the primary arthroplasties had been trauma or trauma sequelae (32%), osteoarthrosis (27%), rheumatic disease (12%), and osteonecrosis (9%). This distribution with the high incidence of trauma patients may be explained by the fact that our facility is a major trauma referral center; most cases included in both groups (60.6%) were referred from other services. The preoperative functional evaluation of patients was performed using the Harris Hip Score,29 and no differences were noticed between groups (average score of 16 in the control group and 18.5 in the study group, P = 0.02).
The evaluation of bone loss on the acetabular and femoral side after removal of the components was made according to the Gustilo and Pasternak classification30 (Tables 1 and 2). Most of the patients in both groups had severe femoral bone loss and mild to moderate acetabular bone loss. The bone loss was similar in both groups, with a trend of worse femoral losses for the study group.
The spacer was similar to a Thompson’s unipolar prosthesis and was made in 2 phases: acetabular and femoral.28 The core of the spacer was a femoral component that was removed during a revision and sterilized (Figure 1); or, ideally, it was a Küntscher femoral nail bent according to the original angle between the neck and the diaphysis of the patient’s femur (Figure 2). We used 1.0 g of vancomycin hydrochloride powder (Vancocina CP®, Eli Lilly, Sao Paulo, SP, Brazil) per package of 40.0 g of acrylic cement (Simplex P®, Striker Howmedica Osteonics, Rutherford, NJ, USA), as recommended by Penner et al31 and by Chohfi et al.32 Typically, 2 packages of cement were used to make the acetabular portion, and 2 to 4 packages were used to make the femoral portion.
Making of a spacer; A – Proximal confection of the spacer, with bone cement (40,0g) mixed with vancomycin (1,0g) and methylen blue covering the metal of a Müller prosthesis; B – Molding of the bone cement in the bone acetabulum, until total drying; C – Distal confection, with the bone cement applied to the stem; D – Stem molding, with sustained movements to avoid fixation to the femoral canal; E – Drying of the bone cement. Note the different blue tones, which represent the two components done; F – Reduction of the spacer into the acetabular cavity.
A – Infected THR after interthrochanteric fracture in a 74 years old patient. Bone loss grade IV at femoral side and I at acetabular side; B – Spacer done with a Küntscher rnail bended to simulate femoral neck angle; C – After two years of second stage done with a wagner prosthesis and massive femoral bone allograft the patient has no signs of infection and a Harris hip Score of 87.
The patients treated without a spacer remained for 3 weeks under skeletal traction to allow fibrosis formation at the Girdlestone resection.
Systemic antibiotics were given for 3 weeks, according to infectious disease protocols. The empiric therapy was 1.0 g of vancomycin and 2.0 g of ceftazidime daily until bacterial isolation. Oral antibiotics were indicated prescribed until 6 months after the first surgical stage, regardless of whether the second stage was/was not completed.
The second stage was done only when erythrocyte sedimentation speed and C-reactive protein were at normal levels. In doubt, or if the increase of the values of the active phase tests persisted, samples taken by intra-articular puncture were collected for culture. If any bacterial growth was noticed, the second surgical stage was not performed and the treatment was considered as failed for this study18,33,34
For both stages, we report the bacteria that were isolated, the surgical length of time, blood reposition, hospital stay, and complications.
Statistical AnalysisWe used the chi-square test (χ2) and Fisher’s exact test to compare the class frequencies between the groups. To compare the magnitudes (quantitative data) between samples of the groups, the Student t test was used for samples presenting parametric distributions, while the Mann-Whitney U-test was used for nonparametric distributions. To compare the magnitude of a same sample at 2 different times (ie, pre- and posttreatment), the Wilcoxon test was used.
RESULTSNone of the patients was lost to follow-up, which was 4 years (range, 2-8.5 years) in both groups. Three patients died of causes unrelated to the treatment after 48 months or more of follow-up; they were included in the study. The final results were as follows:
Thirty patients were treated in the control group, in 2-stages without a spacer. Of these, 1 died of septicemia after first stage and 2 died of hemorrhagic complications after the second stage. These patients account for a 10% mortality related to the treatment without a spacer.
Twenty-three patients underwent reimplantation. In one patient, this was not possible due to technical problems during surgery.
At last follow-up, of the 20 patients with successful implants, 2 reported recurrence of infection within less than 6 months after second stage and 2 had aseptic loosening of the acetabular cup requiring revision surgery. Eighteen (60.0%) patients were infection-free and reported good functional results.
Thirty-eight patients were treated in the study group (with a spacer).
One patient died after pelvic migration of the spacer; this was classified as a method failure.
One patient died of nontreatment-related causes (acute cholecystitis) 4 months after the second stage and was excluded from the study.
Thirty-three patients underwent a new total joint replacement. Two patients had recurrent dislocations and were treated surgically. There were 2 aseptic loosenings: 1 cementless cup was loose requiring revision after 4 months and 1 cemented stem was loose without pain after 4 years.
At the last follow-up, 31 patients (81.5%) had a good functional prostheses with no infectious recurrences.
Bone allografts were used in 61.4% of all cases (35 of 57 second-stage surgeries), which confirms the bone loss stated at first stage surgery
Table 3 shows that the mean duration of the first surgical stage was less in the control group (3 hours 12 minutes, P = 0.02), a finding that shows that the spacer increased the surgical time by 40.1 minutes. At the second stage, the mean duration of the surgery in the study group was virtually 1 hour less (3 hours 22 minutes, P = 0.001).
Table 4 displays hospital stay which was 34.6 days for the control group versus 24.7 days for the study group (P < 0.001), for the 1st stage operation. Once the second stage was performed, a significant difference was noticed in the duration of patients’ hospitalization, with shorter hospital stays for the study group (11.7 days against 8.2 days, P = 0.004). The interval between surgeries was equivalent in both groups, with an average of 226.9 days (range, 70-610 days) for the control group and 162.8 days (range, 60-350 days) for the study group (P = 0.31).
Table 5 displays intensive care unit stay. No differences were noticed between the groups in terms of number of days during which the patients were cared for in the intensive care unit after the first surgical stage, although a trend exists towards a longer time in the hospital in the control group. The use of the spacer allowed patients to have a shorter stay in the intensive care unit after the second surgical stage (1.4 days on the average, as compared to 4.1 days for the control group, P = 0.004).) No difference occurred between groups in the required amount of packed red cell transfusion (Table 6), but less fluid drained in both stages for the study group (Table 7).
Complications are listed in Table 8: Six spacer-related complications occurred: 3 dislocations, 2 pelvic migrations and 1 fracture. Of the dislocated spacers, 2 were from surgeries performed in the beginning of the study. We observed that the necks of these spacers were too valgus, which facilitated lateral migration. Although these 2 spacers remained dislocated, patients did not report any significant pain condition, and it was not difficult to perform the second surgical stage. Other complications were unrelated to the kind of treatment, such as femoral fractures at implant removal (9 cases), delirium (6 cases) and intestinal obstipation (6 cases).
Incidence of complications, by treatment group and totals
| C | Control | Study | Total |
|---|---|---|---|
| Spacer dislocation* | 3 | 3 | |
| Intrapelvic migration of spacer* | 2 | 2 | |
| Spacer fracture* | 1 | 1 | |
| Femoral fracture at implant removal | 4 | 5 | 9 |
| Delirium | 3 | 3 | 6 |
| Intestinal obstipation | 2 | 4 | 6 |
| Deep vein thrombosis | 2 | 2 | 4 |
| Antibiotic allergy | 2 | 1 | 3 |
| Aseptic acetabular loosening | 2 | 1 | 3 |
| Recidivant disclocation | 1 | 2 | 2 |
| Thigh abcess | 2 | 2 | |
| Nerve damage | 2 | 2 | |
| Acute hemorrhage leading to death | 2 | 2 | |
| Aseptic femoral loosening | 1 | 1 | |
| Nausea and sickness | 1 | 1 | |
| Retroperitoneal abscess | 1 | 1 | |
| Genital edema | 1 | 1 | |
| Acute renal failure due to antibiotics | 1 | 1 | |
| Headache | 1 | 1 | |
| Anaphylaxis due to morphine | 1 | 1 | |
| Pneumonia | 1 | 1 | |
| Femoral diaphysial pseudarthrosis | 1 | 1 | |
| Herpes zoster | 1 | 1 | |
| Acute colicystitis, sepsis, and death | 1 | 1 | |
| Acute hemorrhage and hypovolemia | 1 | 1 | |
| Inability to implant prosthesis at second stage | 1 | 1 |
There were 4 complications related to antibiotic therapy: 3 allergic reactions and 1 acute renal failure..
There was 1 case (5.0%) of recurrent dislocation in the control group and 2 cases (6.4%) in the study group.
The incidence of intra- and postoperative complications was not different between groups in both stages. During the first surgical stage, we had problems while removing the fixed femoral components, with femur fractures in 9 cases (23.7%).
Outcome is displayed in Table 9, showing a significantly higher proportion of good results in the study group. The mortality related to the treatment was greater in the control group (10% versus 2.6%), although without statistical significance (P = 0.29).
Outcome of the treatment, by treatment group
| Control | Study | P | |
|---|---|---|---|
| Treament related mortality | 10.0% | 2.6% | 0.29 |
| Final leg length discrepancy (cm) | 2.6 | 1.5 | 0.02 |
| Functional results (points improvement in Harris Hip Score from first consultation to last follow-up) | 39.7 | 54.5 | 0.09 |
| Final results (% of good results at last follow-up) | 60,0% | 81.5% | < 0.001 |
On the average, the leg length discrepancy was 2.6 cm in the control group and 1.5 cm in the study group (P = 0.02).
According to the Harris Hip Score, the final functional outcome was better in the study group, although with no statistical significance (P = 0.09). The average score ranged from 19.3 to 69.0 in the control group and from 19.7 to 75.2 in the study group.
The study group had a better final outcome, with 81.5% having good results versus 60.0% of the control group (P < 0.001).
Recurrence of infection (Table 10). was significantly higher for the control group, where it occurred in 7 patients (23.3%) 7 to 112 days (average, 36.0 days) after the first stage. These patients were treated with successive surgical debridements, muscular flaps, or antibiotic-loaded cement spacers. Three patients (8.1%) reported recurrence of infection after the second stage accounting to an overall infection failure of treatment in 33.3% of the patients.
In the study group recurrence of infection occurred in 2 patients after first stage (5.2%) and in 2 patients (6.1%) after second stage. The overall infection rate was 10.5% in the patients treated with a spacer.
Table 11 displays isolated bacterium species: Gram-positive bacteria were the most frequently isolated during the first surgical stage (68.5%), with prevalence of Staphylococcus aureus (31.5%), coagulase-negative staphilococci (13.7%), and Enterococcus faecalis (13.7%).
Bacteria isolated on cultures during first stage (73 bacteria in 68 patients, 2 patients with 2 or more isolated microorganisms)
| GRAM-POSITIVE BACTERIA | 50 (68.5%) |
| Staphylococcus aureus | 23 (31.5%) |
| Staphylococcus epidermidis, Staphylococcus cohnii and coagulase negative Staphylococci | 10 (13.7%) |
| Enterococcus faecalis | 10 (13.7%) |
| Streptococcus viridans | 3 (4.1%) |
| Streptococcus agalactiae | 2 (2.7%) |
| Corinebacterium sp | 1 (1.4%) |
| Streptococcus mitis | 1 (1.4%) |
| GRAM-NEGATIVE BACTERIA | 23 (31.5%) |
| Escherichia coli | 4 (5.5%) |
| Enterobacter cloacae | 4 (5.5%) |
| Proteus mirabilis | 3 (4.1%) |
| Serratia marcenses | 3 (4.1%) |
| Klebsiella sp | 3 (4.1%) |
| Acinetobacter baumannii | 1 (1.4%) |
| Aeromonas hydrophilia | 1 (1.4%) |
| Citrobacter diversus | 1 (1.4%) |
| Pseudomonas aeruginosa | 1 (1.4%) |
| Providencia sp | 1 (1.4%) |
| Stenotrophomonas maltophilia | 1 (1.4%) |
The use of a spacer reduced the mean duration of the second surgical stage in 1 hour. Reimplantation without a spacer is difficult because it is hard to find the surgical planes, identify the bone structures, and build the bed to accept the prosthesis. An extensive fibrosis results in extended surgery time.
On average, after the first surgical stage, the control group patients stayed in hospital almost twice as long as patients of the study group The use of a spacer reduced the hospital stay, because the skeletal traction period is mandatory to allow healing of the soft parts in patients treated without a spacer.
Although we did not estimate the cost of treatment for patients in this study, the shorter hospital stay, shorter operating room time, and shorter intensive care unit stay of the study group must definitely lead to a lower cost of the treatment with a spacer as compared to that without a spacer.
A technical impossibility of performing permanent arthroplasty during the second surgical stage occurred in 1 patient of the control group, a fact also reported by Fitzgerald35 and Charlton et al.20 This difficulty is typical of the second surgical stage in surgeries without a spacer, in which case it is difficult to dissect the muscular planes and identify bone landmarks.
In the initial trial with the PROSTALAC® Duncan and Beauchamp27 reported 3 cases of spacer dislocation among 15 patients. After 9 years in the revision of 135 cases2,8 only 2 more of such complications exist, showing that the experience acquired with the performance of the method prevents dislocation from occurring. Due to a pelvic migration of a spacer and subsequent injury of the iliac vessels and death of 1 patient, we do not place the spacer as a unipolar prosthesis in patients with acetabular bone weakness, particularly in obese and rheumatoid patients (Figure 3). In this case, we recommend the placement of a cement ball with antibiotics that fills the acetabular cavity and that articulates with the component implanted in the femoral region.
A – Two components spacer made in a massive bone loss in the acetabular and femoral side of a 42 years-old female with Rheumatoid Arthritis, after her second infected revision; B – The same case after one year of follow-up of a second stage with reconstruction of bone loss with allografts, acetabular cement cage and femoral cementless stem.
It is important to note that severe bleeding occurred during the second surgical stage of the control group, with 2 deaths directly related to acute hemorrhage. The dead space left after the removal of components is replaced by a hematoma when a spacer is not placed. This blood collection causes continuous blood loss that is difficult to control and forms the extensive fibrotic tissue found at reimplantation. The dissection of this fibrosis leads to excessive bleeding and to hemorrhagic complications.
Tsukayama et al36 reported 2 infection-related deaths among 98 patients. Fisman et al37 estimate that the mortality associated with infected hip prostheses is 0.4% to 1.2% for 65-year-old patients and 2.0% to 7.0% for 80-year-old patients. During the 3 months that followed the prosthesis removal and the surgical cleaning, mortality doubled for both types of patients.
In both our groups of treatment the dislocation rate was similar to reported data, after the treatment of infected arthroplasties, which is 7.3% to 12.7% of the cases14,16,21,29,30,37,39 regardless of the method employed.
The high incidence of severe femoral loss (up to 19 cases) in the study group demonstrates the long duration of the infectious activity in patients. Severe bone loss makes the treatment even more difficult, since more sophisticated reconstruction techniques are needed during the second surgical stage. It is hard to make a comparison with our series due to the variety of classification systems used. Berry and Chandler38 and English et al18 showed variable bone loss in all cases, and Alexeef et al40 found severe femoral bone loss in 11 cases. Rudelli41 found severe bone loss in 65% of cases treated with 1-stage surgery and bone grafting.
Alexeeff et al40 used massive femoral grafts in 9 cases without infectious recurrence after a 4-year follow-up. The Exeter technique was adopted by English et al18 with infection resolution in 92.5% of 44 cases and good functional results. Hanssen and Osmon42 reported 4 infectious recurrences in 7 patients reconstructed with structured graft of the proximal femur. Rudelli used a homologous bone graft in 36 one-stage treated cases, 9 of them with discharging sinuses, and reached a 88.9% rate of infectious control with a minimum follow-up period of 2 years in the cases with discharging sinuses.41
In our patients, there were no mechanical complications with the massive allografts that were used in 14 patients in the study group and 8 patients in the control group. Maintaining and reconstructing bone stock is fundamental for young patients who will be subjected in the future to new revisions and require a mechanically resistant bone structure for the replacement of worn-out components (Figure 4).
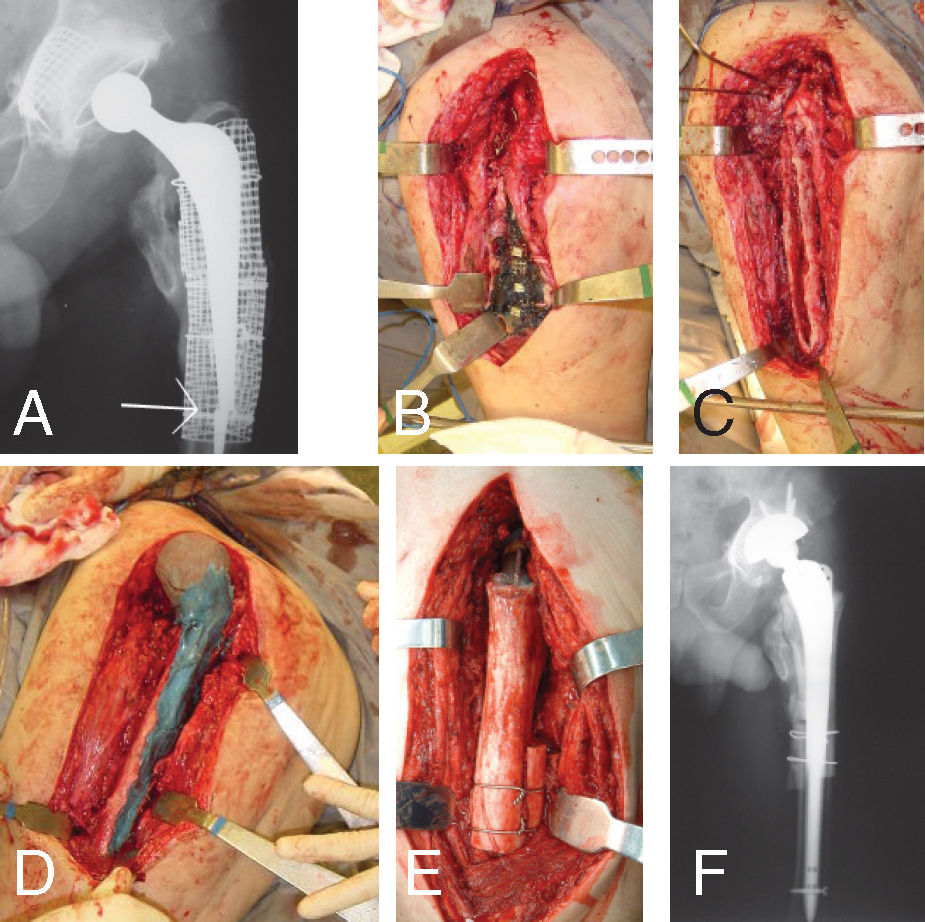
A – Third revision of a THR done in a 45 years old male with Anchilosing Spondylitis. Broken and infected Exeter stem; B – During first stage surgery, there is methylen blue stained at the mesh and dead morselized and impacted bone; C – After removal of the dead bone and infected implants there is a big bone loss classified as Gustilo-Pasternak type IV; D – Spacer introduced and just before reduction into the acetabular cavity; E – Massive femoral allograft at second stage, fixed with cement into the stem. The stem was fixed without cement, with distal interlocking screw and reinforcement with bone strips; F – After three years of follow-up, there is no recurrence of infection and good function (Harris Hip Score is 89).
The tendency towards better functional results in the study group might be due to the advantage that the rehabilitation is performed immediately after the start of the intermediate period. In addition to early rehabilitation in the intermediate period, the use of the spacer led to less surgical aggression in the second surgical stage, with immediate, less painful postoperative handling and early functional recovery.
In both groups the mean leg length discrepancy was acceptable when compared to those of Alexeeff et al,40 who found no discrepancy greater than 3.0 cm when they used a spacer and tissue bank in the second stage, and also to the data of Charlton et al20 who, after the treatment with a spacer achieved full correction in only 50% of the patients.
The identification of Gram-negative bacteria in 22 cases does not contradict the validity of the use of vancomycin added to acrylic cement. Gram-negative bacteria are not sensitive to a single type of antibiotics, a behavior that is adopted by Gram-positive bacteria against vancomycin. The sensitivity spectrum of Gram-negative bacilli varies between aminoglycosides and first to fourth generation cephalosporins and quinolones; therefore, it is difficult to select 1 antibiotic to cover all Gram-negative bacteria. Koo et al43 tried to solve this problem by adding vancomycin, gentamycin, and cefotaxime to the acrylic cement. They found infectious control in 95% of patients, but 4 cases (20%) reported side effects due to the antibiotic, with hepatic dysfunction and medullar depression. In our opinion, the addition of antibiotics to the cement to cover Gram-negative bacteria should be directed and implemented only in case of previous identification of the agent in an appropriately collected culture. This concern is important from the perspective of hospital infection control, since it prevents the conditions favorable to proliferation of multire-sistant bacteria.
Infectious control, both after the first and second surgical stages, was better in the study group—this being the main subject of this article.
After the first surgical stage, the infectious control was poorer in the control group; this is probably due to the colonization of the extended hematoma that occupied the dead space left by the extraction of the metallic implants.
We believe that the follow-up time after second stage is still short and that more accurate analyses should be performed after 5 years of follow-up.
The overall success achieved in both groups was inferior to that reported by other authors who treated infections in 2 stages (72.0% to 97.7%) with a minimum 1-year follow-up period.2,3,9,15,18,37,42,44 Our results are similar to those reported by Hunter and Dandy,45 with 33.3% of infectious control and by Cierny and DiPasquale46 with 64.0%, who treated patients with severe bone loss, as in our case.
All our patients showed discharging sinuses on physical examination, which characterizes the chronicity and severity of infections.44 According to most authors,2,8,15,16,22,28,39,40,47–50 the presence of discharging sinuses contraindicates the performance of a 1-stage treatment. On the other hand, several studies,7,13,14,51 including the communication by Rudelli et al,41 report performance of 1-stage revisions even in the presence of discharging sinuses.
The indications for either 1-stage or 2-stage treatments are not very clear and vary a lot from one treatment center to the next. We agree with Elson,52 who states that a dangerous situation occurs when a given author advocates a single treatment method. There should not be any competitive element in this issue, and it all will depend on the correct comparison of personal results with those found by other investigators. The choice between 1-stage surgery and 2-stage surgery is not so important, since the experienced surgeon knows what is achieved using the selected method.
A 2-stage approach should be used in chronically ill patients, especially those with bone loss and with discharging sinuses. Our overall infection control with both treatments was 73.1%, a value considered desirable in the literature.1–4
CONCLUSIONSWe conclude that the 2-stage treatment of infected hip arthroplasties using antibiotic-loaded cement spacer is superior to the 2-stage surgery performed without the spacer, and that the use of the spacer provides better infectious control, as well as better functional outcomes.