The aim of this study was to evaluate the expression of protein tyrosine kinase 2 and protein tyrosine phosphatase non-receptor type 11, which respectively encode focal adhesion kinase protein and src homology 2 domain-containing protein-tyrosine phosphatase 2, in hematopoietic cells from patients with myelodysplastic syndromes.
METHODS:Protein tyrosine kinase 2 and tyrosine phosphatase non-receptor type 11 expressions were analyzed by quantitative polymerase chain reaction in bone marrow cells from patients with myelodysplastic syndromes and healthy donors.
RESULTS:Protein tyrosine kinase 2 and tyrosine phosphatase non-receptor type 11 expressions did not significantly differ between normal cells and myelodysplastic cells.
CONCLUSIONS:Our data suggest that despite the relevance of focal adhesion kinase and src homology 2 domain-containing protein-tyrosine phosphatase 2 in hematopoietic disorders, their mRNA expression do not significantly differ between total bone marrow cells from patients with myelodysplastic syndromes and healthy donors.
Myelodysplastic syndromes (MDS) encompass a group of hematological disorders characterized by impaired hematopoiesis and a risk of progression to acute myeloid leukemia (AML). Low-risk MDS patients present high levels of intramedullar apoptosis, whereas high-risk MDS patients have impaired cell differentiation and increased cell proliferation (1). Aberrant gene expression is involved in the pathogenesis of MDS and the progression to AML (2). Therefore, studies on the expression of genes involved in cell proliferation, survival and differentiation are important to help elucidate this disease.
Two genes that participate in fundamental cellular processes are protein tyrosine kinase 2 (PTK2) and protein tyrosine phosphatase non-receptor type 11 (PTPN11). PTK2 encodes focal adhesion kinase (FAK), a tyrosine kinase involved in cell proliferation, adhesion and migration (3). FAK is overexpressed in several cancers and its expression usually correlates with a poor prognosis (3). Recent evidences indicate that FAK plays a role in hematopoietic disorders. FAK is upregulated in AML and enhances the migration of leukemic cells from the marrow to circulation, confers drug resistance, and negatively influences the clinical outcome (4). FAK splice variants are abnormally expressed in the primary leukemic cells of AML patients with poor prognosis and induced an increase in the clonogenicity of normal human hematopoietic progenitor cells (5). Moreover, the silencing of this protein in erythroid and myeloid progenitors resulted in a reduced cell growth and survival in response to cytokines, and in a defective activation and expression of antiapoptotic proteins (6).
PTPN11 encodes src homology 2 domain-containing protein-tyrosine phosphatase 2 (SHP2), a tyrosine phosphatase with critical cell properties, including the regulation of proliferation, apoptosis, and differentiation (7). SHP2 expression levels are elevated in AML and are related to the hyperproliferative capacity and the degree of differentiation of primary leukemia cells (8). Animal models lacking SHP2 expression in hematopoietic tissues presented peripheral blood and bone marrow cytopenia (9,10), in addition to increased apoptosis and a reduced quiescence and repopulation capacity of hematopoietic stem cells (10). SHP2 knockdown in normal human cord blood CD34+ cells strongly inhibited cell survival, proliferation, and differentiation in response to growth factor stimuli (11).
Despite the fact that both FAK and SHP2 are upregulated in AML, there are few studies in MDS. Therefore, we aimed to evaluate FAK and SHP2 mRNA expression in bone marrow cells from healthy donors and MDS patients.
MATERIALS AND METHODSBone marrow samplesBone marrow aspirates were obtained from 43 patients diagnosed with MDS (median age: 66 years, range: 16-85 years) before treatment, and from 13 healthy donors (median age: 31 years, range: 18-56 years). This study was approved by the National Ethical Committee Board. Patients' characteristics are described in Table 1. The patients were grouped into low- and high-risk MDS according to the World Health Organization (WHO) (12,13) and French American British (FAB) (14) classifications, and the International Prognostic Score System (IPSS) (15).
Patient characteristics.
| Patient characteristics | Number |
|---|---|
| MDS patients | 43 |
| Gender | |
| Male/Female | 29/14 |
| Age (years), median (range) | 66 (16-85) |
| WHO | |
| Low-risk group: RCUD/RCMD/RARS | 4/20/7 |
| High-risk group: RAEB-1/RAEB-2 | 6/3 |
| AML with myelodysplasia-related changes* | 3 |
| IPSS | |
| Low-risk group: Low-risk/INT-1 | 17/19 |
| High-risk group: INT-2/High-risk | 5/1 |
| Not available | 1 |
| FAB | |
| Low-risk group: RA/RARS | 24/7 |
| High-risk group: RAEB/RAEBt | 8/4 |
| Cytogenetic risk | |
| Low risk | 36/1 |
| Intermediate risk | 3 |
| High risk | 2 |
| Not available | 1 |
| Karyotype | |
| Normal karyotype | 36 |
| -Y | 1 |
| Monosomy 7 | 2 |
| Trisomy 8 | 3 |
| Not available | 1 |
| Number of cytopenic cell | |
| 0/1 | 5/12 |
| 2/3 | 19/7 |
| BM blast (%) | |
| <5% | 31 |
| ≥5 and <10% | 6 |
| ≥10 and <20% | 3 |
| ≥20 and <30% | 3 |
WHO: World Health Organization; RCUD: Refractory Cytopenia with Unilineage Dysplasia; RCMD: Refractory Cytopenia with Multilineage Dysplasia; RAEB-1: Refractory Anemia with Excess Blasts-1; RAEB-2: Refractory Anemia with Excess Blasts-2; AML: Acute Myeloid Leukemia; IPSS: International Prognostic Score System; INT-1: Intermediate-1; INT-2: Intermediate-2; FAB: French American British; RA: Refractory Anemia; RARS: Refractory Anemia with Ringed Sideroblasts; RAEB: Refractory Anemia with Excess Blasts; RAEBt: Refractory Anemia with Excess Blasts in Transformation; BM: bone marrow; *Excluded from the WHO classification analysis.
Bone marrow samples were submitted to RNA extraction using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) after removal of erythrocytes by hemolysis. The reverse transcription reaction was performed using the RevertAid™ First Strand cDNA Synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany). Gene expression was evaluated by qPCR in an ABI 7500 Sequence Detector System (Applied Biosystems, Foster City, CA, USA), using specific primers for amplification of PTK2 and PTPN11 and the suitable housekeeping gene HPRT. Primer sequences are described in Table 2. The relative quantification value of gene expression was calculated using the equation 2-ΔΔCT (16).
Primer sequences and concentrations.
| Gene | Sequence | Concentration |
|---|---|---|
| PTK2 | FW: 5′- GCGTCTAATCCGACAGCAACA -3′RV: 5′- CTCGAGAGAGTCTCACATCAGGTT -3′ | 300 nM |
| PTPN11 | FW: 5′- CCGCTCATGACTATACGCTAAG -3′RV: 5′-AGACCGTTCTCTCCGTATTCC -3′ | 400 nM |
| HPRT | FW: 5′- GAACGTCTTGCTCGAGATGTG -3′RV: 5′- TCCAGCAGGTCAGCAAAGAAT -3′ | 150 nM |
Statistical analyses were performed using GraphPad Instat 5 (GraphPad Software, Inc., San Diego, CA, USA). The Mann-Whitney test was used for comparisons between groups. The level of significance was set at p<0.05.
RESULTSWe observed no differences in PTK2 expression between normal and MDS bone marrow cells (median [range]: 1.00 [0.01-3.39] vs. 1.30 [0.01-8.10]; Figure 1A. PTK2 expression did not differ between low- and high-risk MDS patients according to WHO classification (1.29 [0.01-8.10] vs. 0.60 [0.04-2.20]), IPSS (1.15 [0.01-4.95] vs. 1.79 [0.55-8.10]), or FAB classification (1.26 [0.01-4.95] vs. 1.40 [0.04-8.10]) (Figure 1B-D) and cytogenetic risk (low-risk: 1.09 [0.01-4.95] vs. intermediate/high-risk: 1.40 [0.55-8.10]); all p>0.05. Interestingly, the MDS patient who had presented the highest percentage of bone marrow blasts (23%) also presented the highest levels of PTK2 (6.2-fold above the median of the MDS group).
PTK2 expression in normal and MDS bone marrow cells. (A) PTK2 mRNA expression in total bone marrow cells from healthy donors and MDS patients evaluated by qPCR. (B) PTK2 mRNA expression in low-risk and high-risk MDS patients according to the World Health Organization (WHO) classification, (C) the International Prognostic Score System (IPSS) and (D) the French American British (FAB) classification. Horizontal lines represent median values.
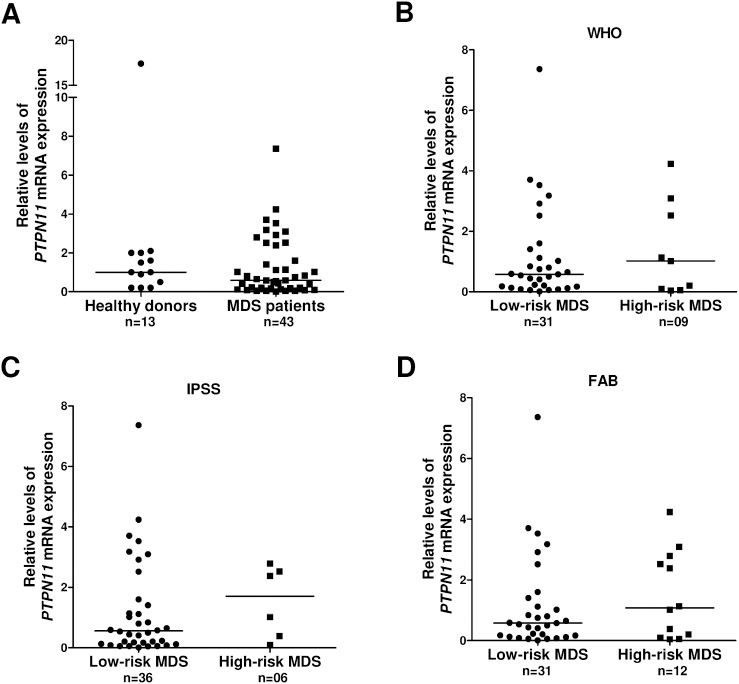
Regarding the analysis of PTPN11 gene, we observed a heterogeneous expression and no significant differences between normal and MDS bone marrow cells (1.00 [0.11-17.39] vs. 0.58 [0.01-7.36]; Figure 2A). The comparison between low- and high-risk MDS patients demonstrated a non-significant increase in PTPN11 expression in the high-risk group according to the WHO classification (0.54 [0.04-7.36] vs. 1.02 [0.05-4.24]), IPSS (0.54 [0.01-7.36] vs. 1.71 [0.10-2.80]) and FAB classification (0.54 [0.01-17.36] vs. 1.08 [0.05-4.24]) (Figure 2B-D). In addition, there was no significant difference in the cytogenetic risk between low- and intermediate/high-risk patients (low risk: 0.54 [0.01-7.36] vs. intermediate/high-risk: 2.52 [0.10-2.80]; all p>0.05).
PTPN11 expression in normal and MDS bone marrow cells. (A) PTPN11 mRNA expression in total bone marrow cells from healthy donors and MDS patients evaluated by qPCR. (B) PTPN11 mRNA expression in low-risk and high-risk MDS patients according to the World Health Organization (WHO) classification, (C) the International Prognostic Score System (IPSS) and (D) the French American British (FAB) classification. Horizontal lines represent median values.
Several studies have reported that FAK and SHP2 are involved in hematopoietic disorders (7,17), which reinforces the need to assess these proteins in MDS. A recent study showed that the increased expression of heat shock protein 90 (HSP90) in mononuclear and CD34+ cells from MDS patients was associated with increased FAK expression and phosphorylation. Moreover, the expression of HSP90, FAK, and pFAK increased after transformation and was related with a poor prognosis or adverse cytogenetics (18). Mesenchymal stromal cells from high-risk MDS patients also presented increased expression and nuclear co-localization of paxillin, pFAK, and HSP90, which correlated with a proliferative advantage of these cells and negatively impacted the clonogenicity of progenitor cells (19). In our study, despite the role of FAK protein expression and activity in MDS cells, we observed no differences in FAK mRNA expression between total bone marrow samples from MDS patients and healthy donors. FAK expression varies according to the hematopoietic cell lineage, and different FAK signaling pathways seem to be triggered according to cell type (3), regulating different aspects of cell behavior, such as proliferation, survival, motility, and interactions between progenitor cells and the bone marrow microenvironment (17). Moreover, FAK phosphorylation is known to play important roles, activating intracellular signaling pathways downstream of integrins and growth factors (17). Therefore, we anticipate that FAK mRNA expression is not abnormal in MDS total bone marrow cells. Furthermore, studies regarding FAK protein expression and activation in isolated hematopoietic cell lineages may help to explain the possible role of this protein in MDS.
PTPN11 expression did not differ between normal and MDS bone marrow cells. We observed an increased PTPN11 expression in high-risk MDS patients compared with low-risk patients; however, the difference was not significant. The small number of high-risk MDS patients may have affected the results; therefore, it is possible that a higher expression of SHP2 is implicated in some cases of MDS, reflecting the heterogeneity of the disease.
In addition to gene expression, mutations and phosphorylation are important SHP2 regulatory events. PTPN11 mutations are associated with hematological disorders. PTPN11 mutations are present in more than 30% of patients with juvenile myelomonocytic leukemia and result in constitutive activation of the Ras signaling pathway and other effectors, deregulating myeloid growth (20). However, PTPN11 mutations do not represent a major molecular event in de novo MDS (21). Phosphorylation of SHP2 follows growth factor or cytokine stimulation and leads to the activation of the PI3K/Akt and RAS/MAPK signaling pathways, which are related to apoptosis and cell proliferation (3,7). SHP2 was found to be constitutively phosphorylated in leukemic cells and in normal hematopoietic cells after mitogenic stimulation, suggesting a correlation between its expression/activation and the hyperproliferative phenotype of leukemia (8). Therefore, as with FAK, it would be interesting to investigate whether the activation of SHP2, rather than mRNA expression, participates in the pathophysiology of MDS.
AUTHOR CONTRIBUTIONSLazarini M and Machado-Neto JA contributed equally to the selection of patients, performed all experiments, analyzed the results, and wrote the manuscript. Archangelo LF, Bigarella CL, and Mendes-Silva BF aided in the quantitative PCR analysis and participated in the writing of the manuscript. Traina F contributed to the selection of patients, clinical follow-up of the patients, analysis of the results, and writing of the manuscript. Ollala Saad ST was the principal investigator.
The authors would like to thank Raquel S Foglio for the English review of this manuscript. This work received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
No potential conflict of interest was reported.