Anemia is a common complication among chronic kidney disease patients on hemodialysis, occurring mostly due to erythropoietin deficiency. This randomized noninferiority trial sought to compare the efficacy and safety of a new epoetin formulation developed by Bio-Manguinhos, a biologics manufacturer affiliated with the Brazilian government, with those of a commercially available product currently used in Brazil (a biosimilar epoetin formulation).
METHODS:The sample size needed to enable demonstration of noninferiority with a statistical power of 85% for a between-group difference in hemoglobin levels of no more than 1.5 g/dL was calculated. In total, 74 patients were randomly assigned to receive the epoetin formulation from Bio-Manguinhos (n = 36) or the biosimilar epoetin formulation (n = 38) in a double-blind fashion. The inclusion criteria were current epoetin therapy and stable hemoglobin levels for at least 3 months prior to the study. The primary and secondary outcomes were mean monthly hemoglobin levels and safety, respectively. The dose was calculated according to international criteria and adjusted monthly in both groups according to hemoglobin levels and at the assistant physicians' discretion. Iron storage was estimated at baseline and once monthly. Clinicaltrials.gov: NCT01184495.
RESULTS:The study was conducted for 6 months after randomization. The mean baseline hemoglobin levels were 10.9±1.2 and 10.96±1.2 g/dL (p = 0.89) in the Bio-Manguinhos epoetin and biosimilar epoetin groups, respectively. During the study period, there was no significant change in hemoglobin levels in either group (p = 0.055, ANOVA). The epoetin from Bio-Manguinhos was slightly superior in the last 3 months of follow-up. The adverse event profiles of the two formulations were also similar.
CONCLUSIONS:The epoetin formulations tested in this study are equivalent in efficacy and safety.
Anemia is a common complication among chronic kidney disease (CKD) patients on hemodialysis, occurring mostly due to erythropoietin deficiency (1). In these patients, replacement therapy with recombinant human erythropoietin (epoetin alfa) leads to decreased transfusion requirements, fewer hospital admissions and improved physical and cognitive performance status, with consequent improvements in quality of life (2-5). In Brazil, epoetin alfa is provided free of charge through the Unified Health System for patients who meet clinical practice guideline criteria (6) and is used by approximately 80-90% (7-9) of the more than 90,000 patients undergoing hemodialysis in the country; 85% of these doses are funded by the government (8).
Since September 2006, Laboratório Bio-Manguinhos (run by the Oswaldo Cruz Foundation, a constituent organization of the Brazilian Ministry of Health) has manufactured and distributed the epoetin alfa dispensed to CKD patients across all Brazilian states. It is of the utmost importance that this product be widely accepted by health care providers and the patient community alike.
The objective of this study was to conduct a head-to-head noninferiority trial comparing the epoetin alfa formulation manufactured by Bio-Manguinhos with a biosimilar formulation previously used by state and municipal departments of health throughout the country.
MATERIALS AND METHODSTo compare the efficacy of the two products containing recombinant human epoetin alfa, a randomized double-blind noninferiority trial design was used. The criteria for inclusion were age >18 years, hemodialysis with subcutaneous epoetin alfa treatment of at least 3 months' duration, baseline ferritin >100 ng/mL, baseline transferrin saturation >20%, Kt/V >1.2 and provision of written informed consent to participate in the study. Patients with hemolytic anemia, myelodysplastic syndrome, multiple myeloma, thalassemia, pancytopenia, sickle cell disease, neoplasms, chronic inflammatory conditions or active bleeding were excluded.
A convenience sampling strategy was used. All CKD patients undergoing hemodialysis at the two participating study centers were considered as potential candidates, as long as they met the aforementioned inclusion criteria.
The patients were recruited and screened at two dialysis centers in Porto Alegre, state of Rio Grande do Sul, Brazil, in May and June 2008. Randomization was stratified by the presence of diabetes and the usual epoetin alfa dose, defined as high (weekly dose >150 IU/kg) or low (weekly dose <150 IU/kg). The patients were randomly allocated to receive epoetin alfa manufactured by Bio-Manguinhos (Fiocruz) or a biosimilar formulation (Alfaepoetina Blausiegel®) by a statistician, who did not participate in the project, using a computer-generated randomization sequence (randomization.com).
The follow-up period was 6 months long. In both groups, all subjects were seen monthly by the study physicians; their test results were reviewed, and their epoetin dose was adjusted as necessary to keep hemoglobin levels in the 11-12 g/dL range. The physicians and all other personnel remained blind to the treatment allocation. Furthermore, also in a blind fashion, all patients received maintenance doses of parenteral iron as necessary to keep serum ferritin >100 ng/mL. Dose adjustment was performed monthly for all subjects. Adverse events were assessed by a chart review, as well as during monthly patient interviews that were conducted by study nurses.
The blinding was performed by two pharmacists, one at each study center, who had sole access to the randomization tables and were exclusively responsible for the receipt, storage and dispensing of the medications. Neither pharmacist played any role in patient care or had any contact with the study center staff involved in patient care. During the shift immediately preceding scheduled epoetin administration, the study pharmacists labeled and preloaded syringes with the appropriate epoetin dose for each patient, which were then stored in an appropriate container under refrigeration. The study nurses administered the drugs without any knowledge of the dose or product.
The sample size needed for a statistical power of 85% and a significance level of 5% was calculated. The ideal sample size was estimated at 70 subjects (35 in each treatment group), taking into account a rate of loss to follow-up of up to 15%. The sample size calculations assumed that both treatments would prove equivalent (with a mean difference in hemoglobin levels no greater than 1.5 g/dL).
The primary outcome of interest was the hemoglobin level and its variation over a 6-month follow-up period in both treatment groups. The secondary outcomes were the epoetin dose and safety variables (as assessed by the adverse event rate).
Statistical analysisThe data were stored in a Microsoft Access database and analyzed in the SPSS 14.0 software environment.
Analysis of variance (ANOVA) was used to compare hemoglobin values and their fluctuation over time between the two treatment groups. The proportion of patients achieving the target hemoglobin range in each group was measured and the chi-square test was used for between-group comparison. Epoetin alfa doses (mean±SD), readjusted monthly in each group throughout the study period, were also compared by ANOVA.
Statistical analyses were conducted on an intention-to-treat basis using repeated-measures ANOVA (with the last observation carried forward). The same method was used for the between-group comparison of other variables, including hematocrit, serum iron, serum potassium and the urea reduction rate (URR).
ETHICSThe study was approved by the Hospital de Clínicas de Porto Alegre Research Ethics Committee (Institutional Review Board-equivalent) and registered at clinicaltrials.gov (Efficacy Study of Two Formulations of Erythropoietin/NCT01184495, http://clinicaltrials.gov/ct2/show/NCT01184495?term = NCT01184495&rank = 1). The study was conducted in accordance with the provisions of the Declaration of Helsinki. All subjects provided written informed consent.
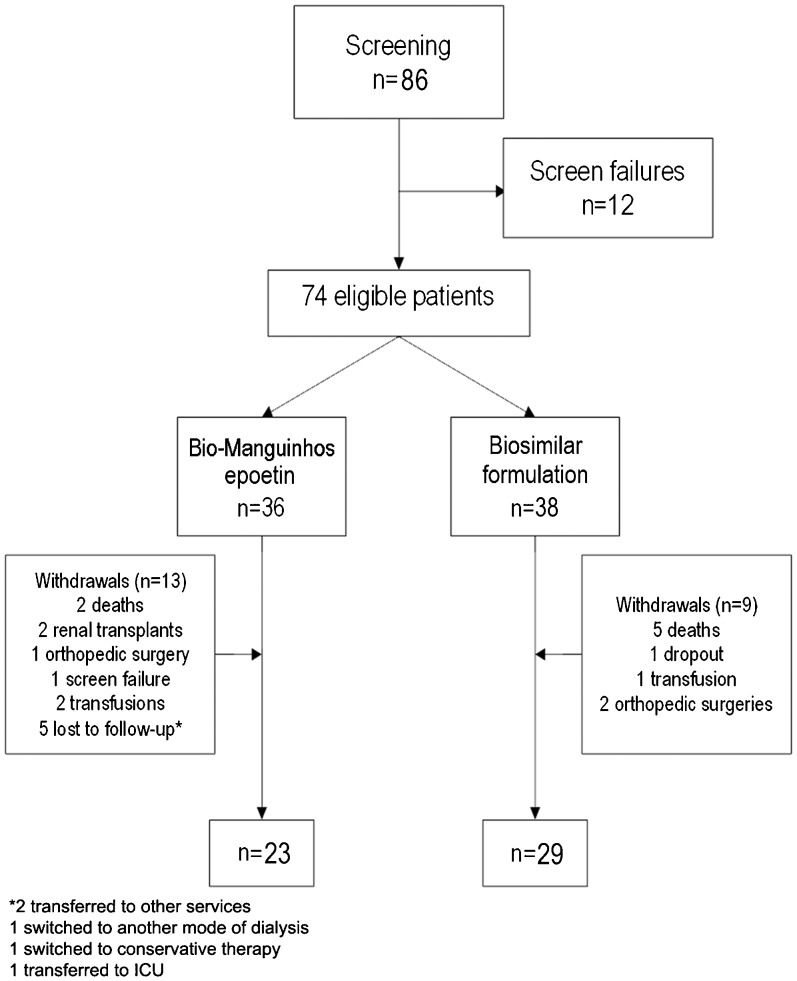
RESULTSA total of 86 patients were screened and 74 were found to be eligible for randomization. Center A randomized 21 patients: 10 to Bio-Manguinhos epoetin (EpoBM) and 11 to the biosimilar formulation (EpoBS). Center B randomized 53 patients: 26 to EpoBM and 27 to EpoBS. Overall, 36 patients were allocated to receive EpoBM and 38 were allocated to receive EpoBS.
Loss to follow-upIn total, 22 patients dropped out of the study over a 6-month period: one due to screening failure, seven due to death, three due to transfusion requirements, three due to surgical intervention, two who were switched to other treatment modalities, three who were lost to follow-up, two who received renal allografts and one who withdrew informed consent (Figure1).
Sample profileThe two experimental groups showed no statistically significant differences in the study parameters at baseline (Table1). There were no significant between-group differences in serum hemoglobin, ferritin, iron or parathyroid hormone (PTH) levels; in the URR; or in the proportion of patients with diabetes. Anthropometric parameters were similar. Female patients were slightly predominant in the EpoBS group, with no statistical significance. The percentage of patients receiving epoetin at doses higher than 150 IU/kg/month was also similar between the EpoBM and EpoBS groups (31.6% vs. 27.8%, respectively; p = 0.721).
Sample profile (by study group).
| Characteristic | Bio-Manguinhos EPO | Biosimilar EPO | p-value |
|---|---|---|---|
| N | 36 | 38 | — |
| Male | 50% (19) | 38.9% (14) | 0.34 |
| Diabetes mellitus | 36.8% (14) | 38.9% (14) | 0.86 |
| Dose >150 IU/kg/week | 31.6% (12) | 27.8% (10) | 0.72 |
| Height (cm) | 164.7±10.2 | 162.3±12.1 | 0.37 |
| Weight (kg) | 66.7±16.5 | 63.6±13.7 | 0.39 |
| Age (years) | 54.6±15.6 | 59.5±13.5 | 0.16 |
| Baseline hemoglobin (g/dL) | 10.9±1.2 | 10.96±1.2 | 0.89 |
| Baseline hematocrit (%) | 34.24±3.8 | 34.6±3.6 | 0.67 |
| Baseline ferritin (ng/mL) | 663.5±384.3 | 623.4±301.2 | 0.62 |
| Iron (μg/dL) | 80.4±37.5 | 68.1±25.0 | 0.10 |
| URR | 70.6±7.2 | 72.2±10.8 | 0.46 |
| PTH (pg/mL) | 434.0±393.6 | 503.7±411.1 | 0.49 |
PTH: parathyroid hormone. URR: urea reduction rate.
There were no significant between-group differences in hemoglobin levels, whether at baseline or during the 6-month follow-up. Throughout the observation period, mean hemoglobin levels remained within the target range of 10-12 g/dL in both groups. Over the final 3 months of the study, mean monthly hemoglobin levels were slightly higher in the EpoBM group, but this difference did not reach statistical significance (p = 0.112). Hemoglobin levels were similar between diabetic and nondiabetic patients, regardless of the epoetin formulation used (p = 0.112).
Epoetin alfa doses (in IU/kg/month), adjusted as necessary from the first month of follow-up to keep hemoglobin levels in the target range of 11-12 g/dL, were also similar between the two groups (Table2 and Figure2). Two-way repeated-measures ANOVA for this variable (epoetin dose) showed that there were no significant differences from month to month throughout the study period. Regarding the mean monthly dose of epoetin alfa used in this study, there was a 25% increase in the EpoBM dose from baseline to month 5 and a 17% increase in the EpoBS dose in the same period. However, this difference did not reach statistical significance. The patients' iron reserves (as measured based on ferritin and iron levels and iron-binding capacity) remained adequate throughout the study period, with no between-group differences (p = 0.314, 0.279 and 0.2, respectively, ANOVA). Serum potassium levels also remained similar between the two treatment groups throughout the study (p = 0.559, ANOVA). Finally, the URR remained within the recommended range (URR>65%) in all patients, with no significant between-group differences (p = 0.31, ANOVA).
Epoetin alfa dose used and hemoglobin values (mean±SD) by patient group.
| Mean monthly total dose of prescribed EpoBM | Bio-Manguinhos Hbbg/dL±SD | Mean monthlytotal dose of prescribed EpoBS | Biosimilar Hgbg/dL±SD | |
|---|---|---|---|---|
| Baseline | 31.6 used doses >150 UI/kg/week | 10.92±1.23 | 27.8 used doses >150 UI/kg/week | 10.96±1.20 |
| Month 1 | 32342.9 | 10.76±1.65 | 30305.8 | 11.07±1.81 |
| Month 2 | 36114.2 | 10.63±1.46 | 27243.7 | 11.03±1.95 |
| Month 3 | 41371.4 | 11.11±1.43 | 31157.9 | 10.91±1.97 |
| Month 4 | 40685.7 | 11.52±1.60 | 34315.8 | 11.05±1.93 |
| Month 5 | 40000.0 | 11.59±1.53 | 35473.7 | 10.98±1.70 |
| Month 6 | 38514.3 | 11.45±1.74 | 36931.5 | 11.07±1.66 |
ANOVA for the change in the mean EPO dose used was not significant; p = 0.361.
ANOVA for mean hemoglobin levels; p = 0.055.
Mean hemoglobin levels during the 6-month follow-up in (A) the groups receiving Bio-Manguinhos epoetin (EpoBM) or a commercially available biosimilar formulation (EpoBS) and in (B) diabetic and nondiabetic patients. (C) Mean monthly doses of epoetin alfa in the EpoBM and EpoBS groups.
Table3 shows the frequency of undesirable signs and symptoms experienced by patients during the study and reported during the monthly interviews. Although adverse effects were relatively common, their incidence was similar between the two treatment groups (p = 0.70). Similarly, we found no significant difference in the incidence of severe adverse events (Table4). Seven patients developed vascular access thrombosis (12 episodes in total). Two patients in the EpoBM group died, compared with five in the EpoBS group; this difference did not reach statistical significance (RR 0.434, 95% CI 0.090-2.096). The cause of death was infection or cardiovascular disease in three cases each and hypoglycemia in the remaining case. No deaths were related to the type of epoetin used.
Number of adverse events reported by patients during monthly follow-up interviews.
| Adverse event | Bio-Manguinhos | Biosimilar | Overall |
|---|---|---|---|
| Fatigue | 52 | 51 | 103 |
| Arthralgia | 64 | 60 | 123 |
| Headache | 37 | 49 | 86 |
| Nausea/vomiting | 37 | 38 | 75 |
| Fever | 4 | 5 | 9 |
| Chest pain | 27 | 28 | 55 |
| Edema | 22 | 20 | 42 |
| Palpitations | 22 | 23 | 45 |
| Diarrhea | 21 | 19 | 40 |
| Dyspnea | 18 | 13 | 31 |
| Pruritus/hives | 43 | 36 | 79 |
| None | 64 | 78 | 142 |
| Total | 411 | 420 | 830 |
This was the first randomized double-blind clinical trial designed to compare the efficacy of epoetin alfa manufactured by Bio-Manguinhos with that of a biosimilar formulation. The chosen comparator product was Alfaepoetina Blausiegel®, which was regularly distributed by the Rio Grande do Sul state health system until December 2006.
Our results show no between-group difference in hemoglobin levels or epoetin alfa doses throughout the 6-month follow-up period. Mean hemoglobin levels remained within the predefined target range throughout the study.
The target hemoglobin range was defined according to recommendations in the current literature (10,11). A more recent study suggested that higher hemoglobin targets are associated with cerebrovascular events and no improvement in quality of life in this patient population (12).
At the end of the study period, one-third of the patients in each group had hemoglobin levels in the 11-12 g/dL range, with no between-group differences. Certain authors (13-15) recommend that hemoglobin values be kept in the range of 10-12 g/dL. In the current study, using this recommendation, 60% of patients in both groups were within the target range every month. These findings are similar to those reported elsewhere in the literature (16-18). In a multicenter study of 24,948 patients receiving hemodialysis at 135 U.S. centers, only 38.4% of patients had hemoglobin levels in the 11-12 g/dL range (19). Another observational study of 987 patients (17) found that mean hemoglobin levels fluctuated between 10.9 and 11.2 g/dL over a 6-month period, with less than 50% of patients remaining within the recommended therapeutic range.
As is the norm for biological variables, hemoglobin ranges exhibit a degree of variation with repeated measurement (20-22). In patients with CKD, this variability is even greater (23) due to a combination of factors, which include patient characteristics, intercurrent events, and clinical practice and care patterns (19,24,25).
In the present study, the epoetin alfa dose was readjusted once monthly, in compliance with Brazilian Health Surveillance Agency Resolution 145, which established that hemodialysis patients should undergo monthly hematocrit and hemoglobin measurements to be consistent with current medical practice. Variability in hemoglobin levels and in the number of patients outside recommended hemoglobin ranges might decrease if doses are adjusted more often (26). From baseline to months 4 and 5, the mean monthly dose of epoetin alfa was higher in the EpoBM group. However, the investigators blindly decided the dose, which might have been the cause of the slightly higher hemoglobin levels noticed in this group at the end of the trial. A longer observation period would be required to enable us to better compare these ‘ups and downs’ between groups.
The leading causes of death in the study were cardiovascular disease and infection, as usually occur in patients with dialysis-dependent chronic renal failure (27,28).
The frequency of signs and symptoms reported by patients during treatment and considered to be adverse effects was similar between the two groups, reflecting the high morbidity of CKD (27,28). These manifestations are part of the uremic syndrome and cannot be fully corrected by current dialysis therapies; renal transplantation is required. Seven hemodialysis-related thrombotic events (associated with vascular access) occurred during the course of the study. Thrombotic events are relatively common in patients undergoing dialysis, occurring at a rate of approximately 0.5-0.8 patients/year (29). The overall A-V fistula thrombosis rate found was 12/74 (16.3%) (30), with no between-group difference (31). This multifactorial adverse event might have been related to the dose of epoetin in both arms, but this relationship still has not been clearly established in the literature (32). Vascular access thrombosis is related to a variety of factors, including the type of access, care and management of the access (33) and higher anemia correction targets (34,35). Furthermore, this thrombosis is even more common in patients with preexisting cardiovascular disease (34). However, due to the small sample size of the present study, we were unable to draw any definitive conclusions regarding this complication.
In conclusion, the recombinant human erythropoietin (epoetin alfa) manufactured by the Immunobiologics Technology Institute of the Oswaldo Cruz Foundation (Bio-Manguinhos, Fiocruz, Brazilian Ministry of Health) exhibited efficacy and safety results comparable to those of a commercially available biosimilar formulation in a sample of CKD patients undergoing hemodialysis. There were no between-group differences in the adverse event rate at any point during the study period. The Brazilian Ministry of Health estimates that domestic manufacturing of epoetin has saved the public approximately US$ 20 million per year. Brazilian manufacturing of a recombinant polypeptide represents massive progress for a country whose biopharmaceutical development technology is at least 30 years behind the state of the art. Incorporation of this technology by a national public laboratory creates the possibility of manufacturing a wide range of other biotechnological products, a possibility that has already been realized at Bio-Manguinhos/Fio-Cruz. Government manufacturing of high-technology pharmaceutical products is vital to countries that distribute and dispense such products through universal health systems, such as Brazil, which has made epoetin freely available nationwide.
ACKNOWLEDGMENTSThe authors would like to thank Dr. Maria de Lourdes Souza Maia and all staff members on the Bio-Manguinhos Clinical Trials team for their support, advice and monitoring of the clinical trial portion of this study.
AUTHOR CONTRIBUTIONSPicon PD conceived the project, was responsible for funding procurement and sponsor approval, study planning and execution, database construction, statistical analysis and manuscript writing. Pribbernow SC was responsible for study planning, care of all subjects, database construction, data qualification, statistical analysis and manuscript writing. Schacher SC and Antunes VV were responsible for patient care at one of the study centers, data collection, database construction and provided assistance to the final draft of the manuscript. Mentz BP and Oliveira FL were responsible for the preparation of the investigational drug, collection of the safety and adverse-event data, safety analysis and provided assistance to the final draft of the manuscript. Souza CMB was responsible for data collection, patient care and provided assistance to the final draft of the manuscript. Schacher FC was responsible for data collection, database construction, data qualification, statistical analysis and provided assistance to the manuscript writing. Prompt CA was responsible for medical coordination of the study, study planning and execution, database construction, statistical analysis and manuscript writing. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
No potential conflict of interest was reported.