Remote ischemic preconditioning (RIPC) is a phenomenon in which a short period of sub-lethal ischemia in one organ protects against subsequent bouts of ischemia in another organ. We hypothesized that RIPC in patients with intermittent claudication would increase muscle tissue resistance to ischemia, thereby resulting in an increased ability to walk.
METHODSIn a claudication clinic, 52 ambulatory patients who presented with complaints of intermittent claudication in the lower limbs associated with an absent or reduced arterial pulse in the symptomatic limb and/or an ankle-brachial index <0.90 were recruited for this study. The patients were randomly divided into three groups (A, B and C). All of the patients underwent two tests on a treadmill according to the Gardener protocol. Group A was tested first without RIPC. Group A was subjected to RIPC prior to the second treadmill test. Group B was subjected to RIPC prior to the first treadmill test and then was subjected to a treadmill test without RIPC. In Group C (control group), both treadmill tests were performed without RIPC. The first and second tests were conducted seven days apart. Brazilian Clinical Trials: RBR-7TF6TM.
RESULTSGroup A showed a significant increase in the initial claudication distance in the second test compared to the first test.
CONCLUSIONRIPC increased the initial claudication distance in patients with intermittent claudication; however, RIPC did not affect the total walking distance of the patients.
Ischemic preconditioning (IPC) was first described in 1986 by Murry et al. (1) as an increase in cellular resistance to myocardial ischemia when the heart is exposed to periods of brief non-lethal ischemia interspersed with reperfusion. In 1993, Pryzklenk et al. (2) demonstrated that an increase in cell resistance to ischemia also occurred in other tissues that were not directly subjected to ischemia. This phenomenon was named remote ischemic preconditioning (RIPC) (3–18). Although some authors question whether RIPC actually occurs (19), many studies have demonstrated the beneficial effects on myocardial cells after transient ischemia is encountered by other tissue (20–22). Cells in organs other than the heart are sensitive to the protective effects that follow ischemia-reperfusion injury of the myocardial tissue or other distant tissues (23–26). Based on evidence of RIPC occurring in other tissues, we predicted that RIPC could occur in patients with intermittent claudication (IC), thereby making the muscles more resistant to ischemia and increasing the ability of these individuals to walk. To test this hypothesis, we performed gait tests in patients with claudication with and without prior RIPC and then compared the initial claudication distance (ICD) and the total walking distance (TWD).
METHODSThis study was performed at the Intermittent Claudication Clinic of the Hospital das Clínicas, Faculdade de Medicina at the Universidade de São Paulo after receiving approval from the local ethics committee. All of the participants signed informed consent forms prior to their enrollment. This study was registered as a clinical trial in the Brazilian Clinical Trials Registry (trial RBR-7TF6TM).
Between January 2009 and May 2011, 52 consecutive ambulatory patients complaining of typical intermittent claudication (IC) in one or both lower limbs that was associated with an absent or reduced arterial pulse in the symptomatic limb and/or an ankle-brachial index <0.90 were recruited for this study. Physical examinations of the upper limbs revealed normal physiology in all the study participants.
The patients were randomly divided into three groups (A, B and C) and underwent two treadmill tests according to the Gardener protocol. Group A was first tested on a treadmill without undergoing RIPC, and then Group A was tested on a treadmill after undergoing RIPC. Group B was first tested on a treadmill after receiving RIPC, and then Group B was tested on a treadmill without receiving RIPC. In Group C (control group), both treadmill tests were conducted without the patient receiving RIPC. The treadmill tests were conducted seven days apart.
The tests were conducted on a treadmill running at a fixed speed of 3.2 km/h, and the required effort was progressively increased (2% increase in the incline every 2 minutes). The initial claudication distance (ICD), which describes the maximum distance a patient can walk without experiencing leg pain, and the total walking distance (TWD), which refers to the distance walked before the patient could not continue walking, were recorded in each test.
RIPC was implemented according to a previously described protocol (22), which is detailed below.
Ninety minutes before exercising on the treadmill, an inflated cuff was positioned on the non-dominant upper limb of the participant three times for five minutes each time. Between each period of inflation, the cuff was deflated for five minutes.
The participants were advised to avoid consuming the following substances, which have been suggested to interfere with the process of ischemic preconditioning (RIPC), within two hours of the test: cilostazol, sildenafil, dipyridamole, glibenclamide, aminophylline, nicorandil, phenylephrine, angiotensin-converting enzyme inhibitors, angiotensin receptor blocker II, statins and steroids, caffeine and alcohol.
Considering the experimental nature of this study, the number of subjects included in each group was calculated based on sample power, as proposed by J. Eng (41).
Statistical analysisThe statistical analyses were conducted using the Statistic 5.1 software (StatSoft, Inc., Tulsa, OK, USA). The data were first tested for normality and homogeneity of variance using the Shapiro-Wilk and Levene tests, respectively.
To compare the general characteristics of the three experimental groups, we used an analysis of variance (ANOVA) of one factor for continuous variables and the chi-squared or Fisher's exact test for categorical variables. To compare ICD and TWD between the three experimental groups, a two factorial ANOVA for repeated measurements was used considering the group (A, B and C) as an independent factor and the time (first or second test) as the repeated factor. When significant effects were observed, the post-hoc Duncan's test was used to verify differences.
Data are presented as relative frequencies, means and standard deviations. For all of the analyses, a significance level of p<0.05 was assumed.
RESULTSA total of 52 patients were analyzed in this study, which included 38 males and 14 females. General patient characteristics are shown in Table 1. No differences were observed between the groups regarding age, race, gender, diabetes prevalence, hypertension prevalence and smoking history. However, Group C had a higher prevalence of dyslipidemia relative to the other groups (p = 0.017).
General characteristics of patients with intermittent claudication.
| Variables | Group A (n = 18) | Group B (n = 16) | Group C (n = 18) | p-value |
|---|---|---|---|---|
| Age (years) | 65.8±7.9 | 60.5±9.5 | 64.0±9.9 | 0.145 |
| Gender (% males) | 72.2 | 69.8 | 77.8 | 0.835 |
| Skin color (% whites) | 72.2 | 75.0 | 77.8 | 0.929 |
| Risk factors | ||||
| Diabetes mellitus (%) | 23.5 | 37.5 | 44.4 | 0.422 |
| Hypertension (%) | 77.8 | 62.5 | 94.4 | 0.074 |
| Dyslipidemia (%) | 38.9 | 25.0 | 72.2 | 0.017 |
| History of smoking | 94.4 | 93.8 | 83.3 | 0.456 |
Values are expressed as the mean ± standard deviation.
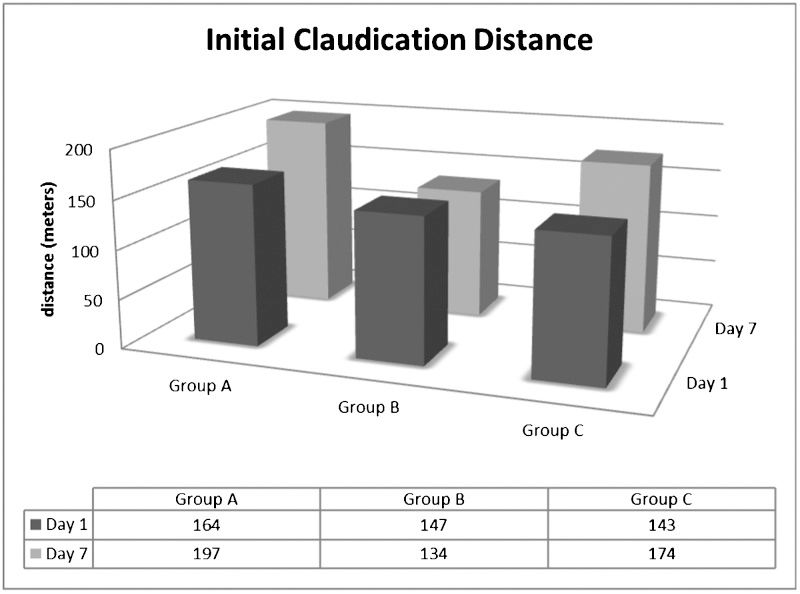
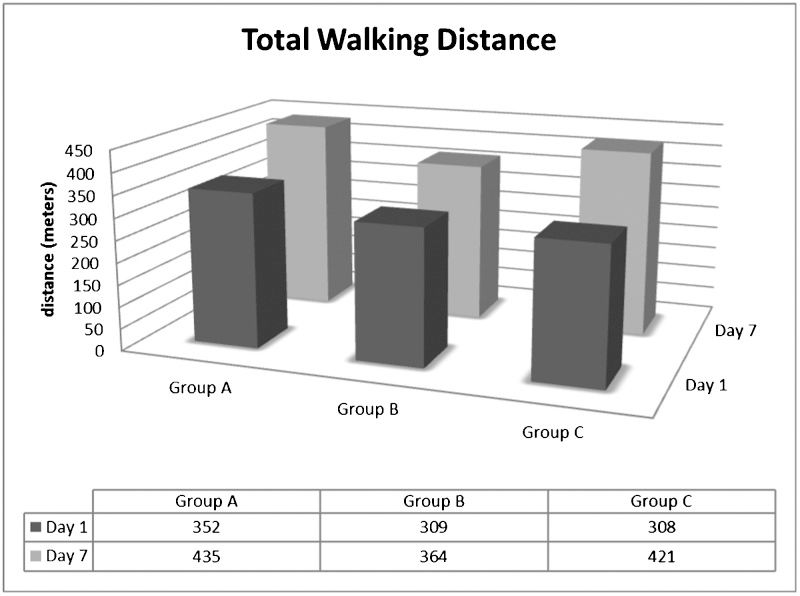
Table 2 shows the effect of RIPC on the walking capacity of the IC patients. Group A and Group C patients had higher initial claudication distances (ICDs) in the second test relative to the first test. However, no differences were found in Group B. Furthermore, the ICD was greater in Group A patients when compared with the other groups. Table 2 also shows that the total walking distance (TWD) was lower in the first test than in the second test for Group A and Group B. However, there were no significant differences between the groups (see Figures 1 and 2).
Effect of remote ischemic preconditioning (RIPC) on initial claudication distance (ICD) and total walking distance (TWD) in the three experimental groups.
| First test | Second test | Group effect | Moment effect | Interaction effect | |
|---|---|---|---|---|---|
| ICD (m) | |||||
| Group A (n = 18) | 164±79 | 197±85∗ | 0.518 | 0.039 | 0.045 |
| Group B (n = 16) | 147±112 | 134±91† | |||
| Group C (n = 18) | 143±114 | 173 ± 135∗† | |||
| TWD (m) | |||||
| Group A (n = 18) | 352±173 | 435 ± 182∗ | 0.692 | <0.001 | 0.595 |
| Group B (n = 16) | 309±168 | 364 ± 218∗ | |||
| Group C (n = 18) | 308±214 | 421 ± 279∗ | |||
Values are expressed as the mean ± standard deviation. ∗Significant difference compared with the first test; †Different from Group A (p<0.05).
Group effect indicates whether a statistically significant difference exists between the groups (A, B and C).
Moment effect indicates whether a statistically significant intra-patient difference exists between the results from the first and second treadmill tests (days 1 and 7).
The interaction effect indicates whether a statistically significant difference exists in both the group and moment effects.
Initial studies analyzing RIPC used animal models in an attempt to show the effects of RIPC by comparing the area of injury in tissues exposed and not exposed to periods of ischemia-reperfusion induced by ligating the arterial feeder vessel. Pryzklenk et al. were the first to demonstrate that small cycles of coronary artery occlusions in dogs protected the myocardial cells from longer periods of ischemia (2). Later, the protective effect induced by ischemia could be transferred to non-preconditioned rabbits that received whole blood transfusions from rabbits that were subjected to RIPC (29). Other studies have shown reductions in the area of myocardial infarction in animals subjected to RIPC induced by the intermittent occlusion of the femoral, renal and mesenteric arteries (30–32). In addition to the cardiac muscle, other organs and tissues have been shown to respond to the protective effect of RIPC, including the lungs, kidneys, liver and skeletal muscle (23),.
Of the few RIPC studies that have been conducted in human subjects, some have shown that RIPC protects endothelial function after ischemia-reperfusion injury (22,36,37), and other studies have shown the protection of myocardial cells in patients undergoing cardiac operations (38,39). For patients undergoing segmental hepatectomy, RIPC before the operation led to reduced liver enzymes during the immediate postoperative period compared with patients in whom RIPC was not performed, but no differences in mortality and morbidity were observed (40). RIPC may be mediated by humoral factors and/or through the neurogenic pathway consisting of an early phase that begins after the completion of preconditioning and persists for upwards of 4 hours, and a late phase that begins approximately 24 hours after RIPC and persists for nearly 48 hours. Several humoral factors appear to be involved in mediating RIPC, including opioids, nitric oxide, adenosine, catecholamines, bradykinin, heat shock proteins, heme oxygenase, tumor necrosis factor-alpha, free radicals, prostaglandins and angiotensin. Organs subjected to ischemia have been shown to activate the protein kinase C intracellular pathway resulting in the nuclear translocation of nuclear factor kappa beta and the subsequent activation of nitric oxide synthesis. Common mechanisms involved in target organs in the early and late stages of RIPC remain unclear; however, some studies have suggested roles for mitochondrial KATP channels and neutrophils.
RIPC is of particular interest with respect to peripheral arterial disease such as intermittent claudication (IC). RIPC could explain, at least partially, the success of physical training in treating patients with IC. The mechanism by which exercise improves the symptoms of vascular-mediated IC remains poorly understood. Furthermore, the initial concept that physical activity promotes increased collateral circulation by stimulating neoangiogenesis lacks definitive evidence (27). Current theories have sought to associate the improvement in walking distance to changes in muscle metabolism induced by exercise (28). Repeated ischemia-reperfusion events caused by physical training could be the stimulus for intracellular biochemical changes leading to a more effective use of oxygen by the muscle and an improvement in endothelial function. This theory is based on the concept of RIPC.
In patients with claudication, a major goal of treatment is to improve the quality of life by increasing the ICD and TWD. Therefore, these parameters were used in our study to evaluate patient responses to RIPC treatment using each patient as his/her own control. We compared the distances walked in each treadmill test with and without RIPC. Because many patients were not used to walking on a treadmill, some patients may have been able to walk a greater distance in the second test after becoming more familiar with walking on the treadmill relative to the first test. Therefore, we divided the patients into two groups that differed in RIPC administration and a control group.
In this study, a significant increase in the ICD was observed in the second treadmill test for Group A and Group C, and this increase was greater in Group A compared to Group C. All of the groups showed a statistically significant increase in the second treadmill test for the TWD regardless of RIPC treatment. These findings suggested that two factors contributed to an increase in walking distance: RIPC and an increase in patient familiarity with treadmill use. Thus, Group A averaged a significantly higher ICD in the second week when compared to the first week. However, in Group B, the factors responsible for increasing the distance walked (RIPC and treadmill familiarization) appeared to antagonize the effect of each other because of the timing of the treadmill test. If RIPC had no beneficial effect on treadmill performance, then the distance walked in the second test should increase regardless of whether RIPC was administered prior to the first test or the second test; however, this was not the case in our study.
The fact that the TWD did not change upon RIPC treatment is most likely caused by ischemic conditioning, which is expected in claudication patients, as ischemia is the cause of the symptoms of claudication. However, as RIPC activates several signaling pathways, we believe that pathways that are not activated or that are partially activated may become fully activated after RIPC, such as the opioid pathway. We suspect that the activation of other signaling pathways by RIPC could explain why the ICD increased, as opioids may increase tolerance to pain.
Future studies should attempt to identify the biochemical agents responsible for the benefits of RIPC in claudication patients, which could allow for the exogenous administration of these factors to claudication patients bringing relief of symptoms. However, prior to the developing therapeutics for claudication patients, evidence confirming the benefits of RIPC in these individuals is necessary, which was the main objective of our study.
Because RIPC did not change the TWD, RIPC did not appear to be useful for claudication treatment, and conventional treatments involving exercise training (walking and/or exercise with load), risk factor control, smoking cessation and drug therapy with aspirin and simvastatin remain the best therapeutic option. However, as patients with claudication are constantly exposed to ischemic conditioning in the lower limbs and because the lower limbs account for a large proportion of the skeletal muscle, claudication patients are an ideal model to research signaling pathways involved with the RIPC effect as well as the triggers of RIPC. RIPC research can lead to the development of therapeutics that could increase cellular resistance to ischemia in patients subjected to acute ischemia, such as trauma patients or major surgery patients.
Additional studies are needed to clarify the effects of RIPC and the mechanism of action through which tissues that are not directly subjected to ischemia benefit upon subsequent ischemic insults.
RIPC increases the initial claudication distance in patients with intermittent claudication. However, RIC does not affect the total walking distance of claudication patients.
AUTHOR CONTRIBUTIONSSaes GF and Zerati AE were responsible for the study concept and design, patient recruitment, data collection and manuscript writing. Wolosker N was responsible for the study concept and design, patient recruitment and writing of the manuscript. Ragazzo L was responsible for patient recruitment, data collection and final approval. Rosoky RM was responsible for the study concept and design, and manuscript writing. Ritti-Dias RM, Cucato GG and Chehuen M were responsible for cardiovascular screening and final approval. Farah B was responsible for statistical expertise and final approval. Puech-Leão P provided medical support during the study and approved the final version of the manuscript.
Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp); grant numbers: 2008/03203-1.
No potential conflict of interest was reported.