To explore the microendoscopic discectomy technique and inclusion criteria for the treatment of recurrent lumbar disc herniation and to supply feasible criteria and technical notes to avoid complications and to increase the therapeutic effect.
METHODS:A consecutive series of 25 patients who underwent posterior microendoscopic discectomy for recurrent lumbar disc herniation were included. The inclusion criteria were as follows: no severe pain in the lumbar region, no lumbar instability observed by flexion-extension radiography and no intervertebral discitis or endplate damage observed by magnetic resonance imaging. All patients were diagnosed by clinical manifestations and imaging examinations.
RESULTS:Follow-up visits were carried out in all cases. Complications, such as nerve injuries, were not observed. The follow-up outcomes were graded using the MacNab criteria. A grade of excellent was given to 12 patients, good to 12 patients and fair to 1 patient. A grade of excellent or good occurred in 96% of cases. One patient relapsed 3 months after surgery and then underwent lumbar interbody fusion and inner fixation. The numerical rating scale of preoperative leg pain was 7.4± 1.5, whereas it decreased to 2.1±0.8 at 7 days after surgery. The preoperative Oswestry disability index of lumbar function was 57.5±10.0, whereas it was 26.0±8.5 at 7 days after surgery.
CONCLUSION:In these cases, microendoscopic discectomy was able to achieve satisfactory clinical results. Furthermore, it has advantages over other methods because of its smaller incision, reduced bleeding and more efficient recovery.
In recent years, microendoscopic discectomy (MED) for lumbar disc herniation has been widely applied in clinical practice due to its minimal exposure and certain efficacy (1-5). The recurrence rate of MED is between 3.5% and 10.8% (6-8). The treatment of recurrent disc herniation usually uses an open technique with a wide exposure for discectomy or lumbar interbody fusion (9-11). MED is seldom used in clinical practice and is considered to be contraindicated for the treatment of recurrent disc herniation due to extensive adhesion in the spinal canal, difficult exposure and potential damage to the nerve root and dural sac (12). Open discectomy with considerable trauma, in essence, also involves adhesions and difficult exposure as well as the complications of dural sac rupture and nerve injury (11). Adjacent segment disease and its influence on lumbar function after vertebrae fusion surgery are the main reasons that patients are unwilling to opt for fusion (13-15). For some patients who previously underwent MED but suffer recurrent lumbar disc herniation in situ, MED reoperation may also be an ideal choice because of the superior stability of the lumbar vertebrae and the absence of significant facet joint degeneration. This study focuses on the choice of cases and the suggested selection criteria and the corresponding technical notes. Through long-term follow-up and subsequent evaluations, the outcomes of this study were used to analyze the availability of selection criteria and the corresponding technical notes.
PATIENTS AND METHODSClinical dataThis is a prospective study. A consecutive series of 25 patients (12 males and 13 females) who underwent posterior MED for recurrent lumbar disc herniation between September 2004 and June 2007 were included (Table1). The ages of the patients ranged from 27 to 62 years old (mean, 50 years old). All segments were at either L4-5 (13 cases) or L5-S1 (12 cases). Before surgery for recurrent herniation, all patients underwent preoperative X-ray imaging, computed tomography and magnetic resonance imaging (MRI) examinations. For those patients without an obvious location sign, electromyography was also performed. All cases were clearly diagnosed based on their clinical manifestations (Figure1).
Summary of the surgical parameters for 25 patients undergoing microendoscopic discectomy for recurrent herniation.
| NO. | Gender | Age | Level of herniation | Side of herniation | Recurrence time (Mon) | Reoperation time (Mon) | Operative time (Min) | Blood loss (ML) | Follow-up (Year) | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 28 | L4/5 | Right | 6 | 9 | 65 | 40 | 3.5 | |
| 2 | M | 33 | L4/5 | Left | 3 | 4 | 60 | 20 | 5 | |
| 3 | M | 36 | L5/S1 | Right | 36 | 40 | 100 | 100 | 1 | |
| 4 | M | 39 | L5/S1 | Right | 14 | 20 | 80 | 80 | 2.5 | |
| 5 | M | 42 | L4/5 | Left | 8 | 10 | 85 | 90 | 3.5 | |
| 6 | M | 55 | L5/S1 | Left | 15 | 18 | 90 | 70 | Recurrence at 3 Mon | |
| 7 | M | 56 | L5/S1 | Right | 23 | 24 | 95 | 90 | 1.5 | |
| 8 | M | 59 | L5/S1 | Left | 10 | 12 | 85 | 60 | 2.5 | |
| 9 | M | 60 | L5/S1 | Left | 9 | 12 | 75 | 90 | 4.5 | |
| 10 | M | 61 | L4/5 | Right | 20 | 23 | 95 | 80 | 2.5 | |
| 11 | M | 61 | L5/S1 | Left | 14 | 16 | 90 | 70 | 2.5 | |
| 12 | M | 62 | L4/5 | Left | 11 | 15 | 80 | 40 | 3.5 | cerebrospinal fluid leak |
| 13 | F | 27 | L5/S1 | Right | 12 | 16 | 60 | 30 | 6 | |
| 14 | F | 38 | L5/S1 | Left | 23 | 24 | 90 | 80 | 2.5 | |
| 15 | F | 39 | L4/5 | Left | 9 | 10 | 75 | 60 | 5.5 | cerebrospinal fluid leak |
| 16 | F | 44 | L4/5 | Left | 8 | 13 | 95 | 80 | 4 | cerebrospinal fluid leak |
| 17 | F | 50 | L4/5 | Right | 7 | 9 | 80 | 70 | 3 | |
| 18 | F | 51 | L4/5 | Left | 12 | 15 | 95 | 80 | 2 | |
| 19 | F | 53 | L5/S1 | Left | 14 | 16 | 95 | 90 | 2.5 | |
| 20 | F | 56 | L4/5 | Right | 5 | 10 | 75 | 80 | 2 | |
| 21 | F | 57 | L5/S1 | Left | 20 | 23 | 100 | 90 | 1.5 | |
| 22 | F | 59 | L4/5 | Right | 9 | 12 | 80 | 40 | 2 | |
| 23 | F | 60 | L4/5 | Left | 8 | 13 | 95 | 30 | 3.5 | |
| 24 | F | 60 | L5/S1 | Left | 16 | 18 | 95 | 90 | 1.5 | |
| 25 | F | 61 | L5/S1 | Left | 18 | 19 | 85 | 40 | 1.5 | |
| Analysis | M (12); F (13) | 50 | L4/5 (12) L5/S1 (13) 16.04 84.867.6 2.916666667 | R (9); L (16) | 13 | 16 | 85 | 68 | 3.0 | / |
Typical case: Female, 27 years old, relapsed one year after microendoscopic discectomy for L5S1 disc herniation. The lumbar disc herniated, along with repeated annulus fibrosus rupture in situ (see arrows). The X-ray showed fair stability of the lumbar spine, no significant decrease in the intervertebral space, and unobvious facet joint degeneration. Microendoscopic discectomy was applied along the original surgical approach (line shown), and the exposed herniated disc was removed from the interior facet joint.
All patients underwent conservative treatment for 1–6 months. Treatment measures included position restriction, nonsteroidal anti-inflammatory drug administration and physical therapy. Surgery was performed when the treatment efficacy was not obvious or the symptoms suddenly worsened. For example, leg pain limits normal activities; there is weakness and numbness in leg; it is difficult to walk and stand; medication and physical therapy are ineffective; the surgery is recommended. Inclusion criteria for this study were as follows: the relatively intact facet of joint was retained with a surgical excision no more than a half and no serious back pain, lumbar spine instability, lumbar disc endplate inflammation, or lumbar disc endplate damage were present.
Surgical techniqueThe surgical technique used was the same as the standard MED treatment for primary lumbar disc herniation but included scar dissection.
Surgical instrumentA microendoscopic discectomy type II (Sofamor Danek, USA) instrument, a matched image pick-up system, bayonetted forceps, nucleus pulposus forceps, and a curved curette were used for the procedure.
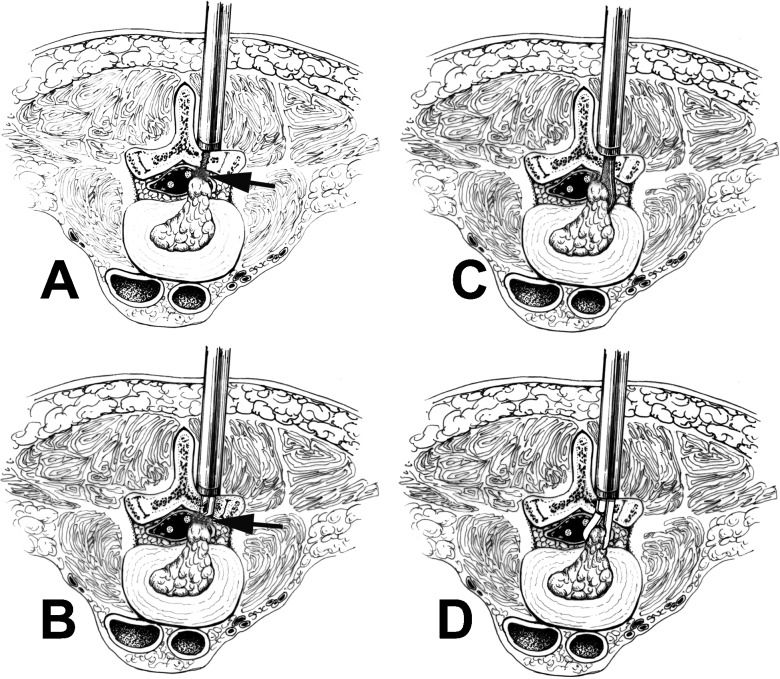
Surgical procedureThe surgery was performed under epidural anesthesia or general anesthesia with the patient in the prone position, with flexed hips and knees and the patient's abdomen in the suspended position to reduce nerve root tension and prevent bleeding caused by spinal venous plexus expansion (Figure2) (1). For the incision, the body surface corresponding to the diseased disc coronal axis was located using an anteroposterior X-ray and the surface position line was taken as the center. A 1.5-cm longitudinal incision was then laterally opened 0.5 cm from the spinal midline (2). To reveal the laminar space, the lumbodorsal fascia was incised 1–1.5 cm along the edge of the spinous process, the paraspinal muscles were moved with a 1-cm wide periosteal dissector and the secondary expansion tube was inserted and propped against the vertebral plate. This opening was progressively expanded and the operating channel was then installed; the scar tissue and residual muscle tissue between the vertebral plates were cleaned and the bone border of the last fenestration operation was revealed (Figure2A) (3). To enter into the spinal canal and incise, the adhesion between the bone border and scar tissue was dissected with a nerve dissector or Kerrison based on the anatomical and imaging features. This procedure was repeated against the spinal canal bone surface with a 90-degree short spherical probe or dissector and then the adhesion was rotated, probed and dissected. Next, the scar tissue was carefully removed or resected and the window was expanded to the desired range with a Kerrison (Figure2B) (4). To reveal the disc herniation, the anatomical relationship between the dural sac and the nerve root was carefully identified and then the adhesion, the inward retracting nerve root and the dural sac on the surface of the disc herniation were dissected with a 90-degree short spherical probe or dissector. The nerve root and dural sac were then fixed and protected with a nerve retractor and wire retractor with a suction tube to clearly expose the herniation (Figure2C) (5). The intervertebral disc herniation was then incised and the broken disc tissue was removed; to avoid harming the nerve roots, the availability of the corresponding segmental nerve root and the anatomical variations of the corresponding segments of the nerve roots and upper nerve root were identified (Figure2D) (6). The corresponding segmental nerve root lateral recess and the upper nerve root canal were then explored and decompressed by ensuring that a 90-degree, long spherical probe or dissector could be placed inside and easily moved up and down without any blockage. Special attention was paid as excessive manipulation may cause serious damage to the intervertebral joint, thereby affecting the postoperative inter-vertebral stability (7). Then, the spinal canal was explored and cleaned. Whether the disc herniation was a free or prolapse type and whether free nucleus pulposus tissue residue was present in the periphery of the dural sac, nerve front, lateral recess and nerve root canal was determined using a 90-degree, long spherical probe or dissector. The ossification growths on the posterior lumbar edge and the protruded annulus fibrosus attachment, which cause compression of the nerve roots, were then removed. This step often caused bleeding. The spinal canal and intervertebral space were then washed with 300–500 ml of ice-cold normal saline; if there were slight ruptures in the dural sac and nerve root sleeve portion, a cotton sheet was placed over them during the operation. After detecting and cleaning the spinal canal, a gelatin sponge was placed over it. If there was considerable damage to the dural sac and nerve root sleeve portion, MED was changed to open surgery to repair the damage.
The technical note for repeated microendoscopic discectomy for recurrent lumbar disc herniation is displayed using a schematic diagram. A: The paraspinal muscles are first moved in the original incision and the secondary expansion tube is inserted and propped against the vertebral plate. Then, the operating channel is progressively expanded and installed, the scar tissue and residual muscle tissue between the vertebral plates are cleaned and the bone border of the last fenestration operation is revealed. B: The adhesion between the bone border and scar tissue is dissected (see arrows) with a nerve dissector or Kerrison based on anatomy and imaging features and the scar tissue is further carefully removed or resected. The window is then expanded to the desired range with a Kerrison. C: The anatomical relationship between the dural sac and nerve root is then carefully identified, the adhesion is then dissected and the nerve root and dural sac are inwardly retracted on the surface of the disc herniation with a 90-degree short spherical probe or dissector. Then, the herniation can be clearly exposed. D: The intervertebral disc herniation is incised and the broken disc tissue is removed.
The routine postoperative anti-inflammatory treatment with a glucocorticoid and dehydration treatment with mannitol was followed for 5–7 days. The patients were provided back muscle training after 3–5 days and ambulation with a leather waist girdle after 5–7 days. The patients resumed their daily activities without a waist girdle 3 weeks later. Outpatient follow-up visits were conducted in the 1st, 3rd, 6th, 9th and 12th months after surgery and every 6 months thereafter. The primary outcomes included a numerical rating scale (0-10) and lumbar function Oswestry disability index (ODI) scoring (0% represents no pain and no disability and 100% represents the worst possible pain and disability). The secondary outcomes included the operative time, blood loss, postoperative analgesics, reoperation and complication rates, and MacNab criteria (16). X-ray examinations in the L-spine anteroposterior position, L-spine lateral position and L-spine lordotic kyphotic position were routinely taken and an MRI was performed annually or when nerve symptoms occurred. Nonsteroidal anti-inflammatory medication was administered if necessary.
All data are presented as the mean ± STD and subjected to a paired Student's t test (numerical rating scale) and one-way ANOVA (ODI scores) with the SPSS version 11.0 software package. Differences were considered significant if p<0.05.
RESULTSThe operation time ranged from 60 to 100 min (mean, 85 min). The average blood loss was 68 ml (range: 20 to 100 ml). No nerve root or cauda equina injury was observed. A small dural tear, observed in three patients, was covered with a gelatin sponge without any repair. No postoperative cerebrospinal fluid leakage occurred. All incisions healed by first intention.
Follow-up visits took place over a 1–6-year period (mean, 3 years) and were undertaken in all cases. The clinical outcomes were graded using the MacNab criteria. A grade of excellent was given to 12 patients based on the resolution of preoperative symptoms, normal results in the straight leg raising test, good lumbar segment motion and partial nerve function recovery. These patients were able to return to normal work. A grade of good was given to 12 patients who displayed relief of their preoperative symptoms, occasional pain and somewhat improved functional capacity. These patients were able to return to modified work. A grade of fair was given to one patient who displayed an improvement of symptoms but still experienced pain and was unable to work. A grade of excellent or good occurred in 96% of cases. One case was not included in the follow-up data because the patient relapsed 3 months after surgery and then underwent lumbar fusion. X-rays showed fusion in the operated segment at 3 months after the operation.
The numerical rating scale of preoperative leg pain was 7.4±1.5; this value decreased to 2.1±0.8 after surgery, giving a statistically significant difference (p<0.05). The preoperative ODI of lumbar function was 57.5±10.0, whereas it was 26.0±8.5 at 7 days after surgery. Follow-ups showed that lumbar function was significantly improved after surgery and recovered to the optimal condition 3 months after surgery, with no significant changes thereafter. There was also a statistically significant difference between the preoperative and postoperative ODI scores (p<0.05) (Figure3).
Imaging revealed the degenerative tendency of the treated segment over time. X-ray examination showed the decreasing height of the treated intervertebral space, facet joint degeneration and lumbar instability. MRI revealed degeneration of the intervertebral discs in the treated segments as well as mild herniation.
DISCUSSIONThe major problems with using posterior MED for recurrent lumbar disc herniation are the limited surgical field, the adhesion caused by surgical scarring and complications, such as potential dural tearing and nerve root injury (17-22). All of the factors mentioned above might be risks of failing to relieve the symptoms caused by recurrent lumbar disc herniation. Once the surgery fails, reoperation is required. To reduce the complications caused by reoperation, most surgeons choose to widely expose the recurrent herniated disc lesion and reconstruct lumbar spine stability by fusion rather than by MED as MED is considered to be contraindicated in the treatment of recurrent lumbar disc herniation (12). However, the clinical observations of the patients in this study showed that MED for recurrent lumbar disc herniation in situ still has a good effect if appropriate patients are strictly selected.
All patients in this study were diagnosed by symptoms, signs and imaging evidence. For patients with unobvious signs of localization, further electrophysiological examination was conducted. For patients with recurrent lumbar disc herniation in situ, the technical difficulty of MED for re-discectomy lies in the dissection of scar tissue, the identification and exposure of the extraspinal structure, the avoidance of nerve root and dural injury, and the minimization of facet joint interior bone resection. Regarding the pathological characteristics, most recurrent disc herniation in situ cases are of the disc prolapse type, especially those recurrences that occur a short time after MED, most of which are caused by omission of the free nucleus pulposus within the intervertebral space and its repeated dislocation into the spinal canal (23). Prolapsed intervertebral disc tissue was limited to annulus fibrosus rupture due to scar tissue adhesions, thus allowing easier elimination of nerve root compression during surgery without a wide exposure. Because the scar tissue is encountered first, reaching the herniation of the nucleus pulposus is the key problem. In the conventional view, the scar tissue should be completely cleared. However, clearing the scar tissue from the neural tissue easily leads to spinal dural fracture. The incidence of spinal dural fracture during open surgery is 8–18% (24), whereas that of MED is 13% (19), indicating similar incidences. Scarring generally does not generate nerve compression symptoms, but a small amount of herniated nucleus pulposus tissue could cause nerve compression if the nerve tissue adheres to the scar tissue. Even if the scar tissue has been cleared, the nerve tissue could still be at risk for adhesion caused by repeated postoperative scarring. Therefore, in this series of surgical operations, the dissection of the scar tissue from the nerve was not emphasized. In contrast, scar tissue adherence to the nerve tissue can protect the nerve tissue when the nucleus pulposus is excised from it. All dural rupture cases in this series occurred due to the attempt to dissect scar tissue from neural tissue (3 cases, 12% incidence), with no significant difference compared to other studies (19). The surgeon discovered that the herniated nucleus pulposus mostly gathered in the annulus fibrosus rupture (the lateral or ventral portion of the nerve roots). Therefore, the herniated nucleus pulposus could be accessed from the medial border of the facet joint and vertebral pedicle. It is relatively safe to dissect scar tissue from the bone walls. Based on dissecting or cutting the scar tissue from the bone border, the surgeon should fenestrate the bone tissue to the desired range without damaging the dural region and nerve to explore and dissect the adhesion tissue, reveal the dural sac and nerve root, further remove the prolapsed intervertebral disc tissue and clean up the loose nucleus pulposus tissue within the intervertebral space to achieve complete decompression and relapse prevention. Cleaning up the intervertebral disc tissue within the intervertebral space is an effective way to prevent recurrence, but excessive removal should be avoided to prevent significantly decreasing the height of the vertebrae interval. Based on our experience, disc tissues that can be easily removed with a nucleus pulposus clamp must be removed, whereas a tough and tenacious nucleus pulposus cannot be forcibly removed. For patients with recurrent disc herniation, the operation time was 85 min, which did not significantly differ from that of open surgery (88.9 min) (25). In this study, 12 cases had excellent outcomes (48%), 12 cases had good outcomes (48%), 1 case had a fair outcome (4%) and no patients had poor outcomes. One case relapsed 3 months after surgery and the recurrence rate was 4%. Postoperative leg pain and lumbar function were significantly improved with an excellent rate of 96%, which reflects the advantages of MED, including less trauma and the rapid relief of leg pain (5-7,12,17-23).
However, for patients with severe lower back pain, lumbar instability and a severe degenerative intervertebral disc with clear end-plate osteochondritis, we do not recommend this therapy because it may aggravate the symptoms of lower back pain after surgery or induce severe back pain instead of relief. The postoperative imaging supplied evidence demonstrating that the treated intervertebral disc degenerated over time although MED was less invasive. Patients who underwent open surgery to remove the nucleus pulposus with wide exposure in their first surgery have no good indicators; therefore, using MED to treat recurrence is of low value. Based on our inclusion criteria, the outcomes of this study showed that most patients possessed good lumbar function based on postoperative ODI scoring, suggesting that our inclusion criteria were available. Of note, the number of cases in this study was small and this study lacked a comparison with other surgeries. Further design of a multi-center randomized controlled trial may provide some additional convincing evidence.
As long as this technique is applied appropriately and reasonably, MED may represent a good treatment option for recurrent lumbar disc herniation in situ that could avoid the complications of bone graft fusion and cause less trauma compared with open surgery. However, as its operation technique is more difficult, there may be a relatively steep learning curve for surgeons.
This work was supported by The Clinical Great Foundation of Third Military Medical University (2012XLC01).
AUTHOR CONTRIBUTIONSHou T wrote the manuscript, collected data and conducted the surgeries. Zhou Q and Xu J edited the manuscript. Dai F, Luo F, He Q, Zhang J and Zhou Q conducted the surgeries.
No potential conflict of interest was reported.