: To verify the accordance of functional and morphometric parameters during the development of emphysema.
METHODS: BALB/c mice received a nasal drop of either papain or saline solution and were studied after 1, 3, 15, 28, and 40 days. Functional parameters, such as airway resistance, tissue damping, and tissue elastance, were analyzed. To evaluate the structural changes and possible mechanisms involved in this disease, we measured the mean linear intercept, the volume proportions of elastic and collagen fibers, the number of macrophages, the numbers of cells expressing metalloprotease 12 and 8-isoprostane in lung parenchyma.
RESULTS: We only observed decreases in tissue elastance and tissue damping on the 28th day, with a concomitant increase in the mean linear intercept, indicating the presence of emphysema. However, only the mean linear intercept values remained increased until the 40th day. The volume proportion of collagen fibers was increased from the 15th day to the 40th day, whereas the volume proportion of elastic fibers was only increased on the 40th day. The number of macrophages increased beginning on the 1st day. The expression of metalloproteinase 12 was increased from the 3rd day until the 40th day. However, 8-isoprostane expression was only increased on the 1st and 3rd days.
CONCLUSIONS: In this study, morphometric parameters were found to be more reliable for detecting the presence of emphysema than the functional parameters measured by respiratory mechanics. Further investigations are necessary to understand how the extracellular matrix remodeling observed in the lung parenchyma could be involved in this process.
Pulmonary emphysema is a major component of chronic obstructive pulmonary disease (COPD), and it is an important cause of morbidity and mortality worldwide.1 It is characterized by an enlargement of the respiratory air spaces and is associated with a breakdown and reorganization of the connective tissue fiber network. Measurements of respiratory mechanics and lung morphometry have been extensively used to evaluate pulmonary changes after the induction of emphysema in experimental models.2,3
Many mechanisms are thought to be involved in the development and progression of emphysema, including protease/anti-protease imbalance, inflammation, oxidative stress, and extracellular matrix remodeling.4 In an attempt to distinguish among these various mechanisms, some experimental models have been designed to reproduce emphysema-induced changes similar to those found in humans. For example, the exogenous administration of proteases, and particulates and exposure to cigarette smoke have been used to induce emphysema in animal models.5 The administration of proteases produces rapid and significant air-space enlargement and macrophage accumulation in rodent and dog lungs.6 The number of alveolar macrophages increases in emphysematous lungs, particularly at sites of disease activity, and these cells are thought to be chiefly responsible for producing metalloproteinase 12 (MMP12), which exhibits proteolytic activity, cleaves elastin, generates fragments that are chemotactic for monocytes, and perpetuates macrophage accumulation and lung destruction.7
The destruction of alveolar walls in emphysema, with consequent alterations in the elastic and collagen fiber networks, alters lung viscoelastic properties, which are evaluated by functional parameters, such as lung elastance measurements. Many studies use the constant-phase model to describe the input impedance of the respiratory system from data obtained by the forced oscillation technique (FOT) and to calculate tissue elastance (Htis), tissue damping (Gtis), and airway resistance (Raw). Raw includes contributions from both the chest wall tissues and the pulmonary airways; Gtis and Htis characterize the viscoelastic properties of the respiratory tissues.8–10
In addition, lung morphometry has been extensively used to verify pulmonary changes in animal models of emphysema, where it remains one of the most reliable techniques for evaluating alveolar destruction.11 An increase in the mean linear intercept (Lm) correlates with alveolar enlargement and parenchymal wall destruction in mice and rats with lung emphysema.12 Moreover, many studies have found an increase in the total number of collagen and elastic fibers, which indicates extracellular matrix remodeling in this disease.3,13
However, the results obtained by these different methodologies are not always consistent because structural changes follow different courses than functional changes in emphysema.14 In a previous study, we found an increase in Lm and in the amount of elastic and collagen fibers in the parenchyma two months after papain administration in mice,15 but no significant differences were detected in the animals' respiratory mechanics. The distribution of emphysema throughout the lung is associated with the altered composition of elastic and collagen fibers and might be important for explaining these results.
To verify how respiratory mechanics and morphometry parameters change during the development of emphysema in mice, we examined whether changes in oscillatory mechanics were in accordance with changes in parenchymal structures at different time intervals after protease administration in mice.
MATERIALS AND METHODSThis study was approved by the Institutional Review Board. Six- to eight-week-old male BALB/c mice were used. All the animals received care in compliance with the “Principles of Laboratory Animal Care” published by the National Institutes of Health.
Induction of emphysemaSeventy BALB/c mice (23-25 g) received a nasal administration of 50 μL of a 10-mg/mL papain solution (20 mg/kg, 6,000 UI/mg; Valdequimica, São Paulo, Brazil). This dose of papain has previously been shown to induce pulmonary emphysema in mice.12,15 The control mice received 50 μL of 0.9% NaCl (saline), the vehicle used for the papain.
Experimental groupsAfter the administration of papain (group P) or the same amount of saline solution (group S), mice from each group were randomly assigned to five subgroups that corresponded to the days on which they were euthanized after nasal administration (P 1, 3, 15, 28, and 40 as well as S 1, 3, 15, 28, and 40).
Assessment of respiratory mechanicsThe animals were deeply anesthetized by an intraperitoneal injection of thiopental (70 mg/kg), tracheostomized and then connected to a ventilator for small animals (Flexivent, Scireq, Montreal) with a tidal volume of 10 mL/kg (120 breaths/min) and a positive end expiratory pressure (PEEP) of 5 cm H2O. The experimental data from the forced oscillation technique were obtained only after the animals had been paralyzed with pancuronium bromide (0.2 mg/kg). On the basis of a previously described model,16 respiratory mechanics were characterized by the following parameters: Raw, Gtis, and Htis.
Sample preparationAt the end of the respiratory mechanics evaluation, the abdominal wall was opened, and the animals were exsanguinated via the abdominal aorta. The thoracic cavity was then opened, and the lungs were removed. Both lungs were fixed using 10% buffered formalin infused through the trachea at a constant pressure of 20 cm H20 for 24 hours and were then embedded in paraffin. Lung tissue sections (5 μm) were stained with H&E, Sirius red, or resorcin-fuchsin for lung structure analysis, collagen fiber evaluation or elastic fiber evaluation, respectively.
ImmunohistochemistryThe sections were deparaffinized and hydrated. After blocking endogenous peroxidase activity, antigen retrieval was performed with either high-temperature citrate buffer (pH = 6.0) or trypsin. The following primary antibodies were used: goat polyclonal anti-mouse MMP12 (1:1,000, Santa Cruz Biotechnology, CA, USA), anti-mouse macrophage marker Mac-2 (1:10,000, clone M3/38, Cedarlane, ON, Canada), and polyclonal goat anti-8-epi-PGF2a (1:1,200, Oxford Biomedical Research, Oxford, England). The Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, USA) provided the secondary antibody, and 303-diaminobenzidine (DAB, Sigma, St. Louis, MO, USA) was used as the chromogen. The sections were counterstained with Harris hematoxylin. For the negative control, the primary antibody was omitted from the procedure, and BSA was used instead.
MorphometryFor conventional morphometry, an eyepiece with a coherent system of 50 lines, 100 points, and a known area attached to the microscope ocular were used. The mean linear intercept (Lm), an indicator of the mean alveolar diameter,17 was assessed in 20 non-overlapping fields of lung parenchyma per animal at 200x magnification. The volume proportion of collagen or elastic fibers in the alveolar tissue was determined by dividing the number of points contacting the collagen or elastic fibers by the total number of points contacting the alveolar septa.18 All of the measurements were performed on 10 non-overlapping fields in each animal at 400x magnification.
The numbers of macrophages and MMP12-expressing cells in the alveolar parenchyma were also assessed by point-counting. Using the eyepiece (62,500 μm2 at 400x magnification), the number of points in each field contacting alveolar tissue was counted. The alveolar tissue area in each field was calculated as the number of points contacting alveolar tissue as a proportion of the total grid area. The number of positive cells within the alveolar tissue area was counted, and the results were expressed in cells/μm.2,19 The expression of 8-isoprostane was assessed using a digital analysis system and specific software (Image Pro Plus v. 4.5 for Windows, Media Cybernetics, USA). Sections were stained with an 8-isoprostane antibody and captured using a microscope (E200, Nikon, Japan) connected to a camera (Infinity 2-1 Monochrome CCD camera, Lumenera, Canada), and the images were fed into a computer. The isoprostane-stained area (%) was expressed as the amount of isoprostane in a specific frame relative to the total area of tissue within that frame.
Statistical analysisAll of the data are expressed as means ± SD. Statistical analyses were performed using SigmaStat software (SPSS Inc., Chicago, IL). Student's paired t-test was used at each time point (1, 3, 15, 28, and 40 days) to compare the papain and saline groups. A p-value of less than 0.05 was considered to be significant.
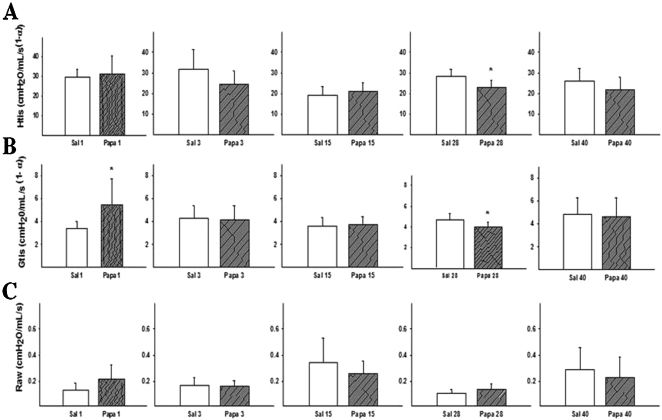
RESULTSMechanics AssessmentThe mean (±SD) values of Raw, Gtis, and Htis measured at various times after papain administration are presented in Figures 1A, 1B, and 1C, respectively. When comparing the papain group (P) with the saline group (S) at each time point, an increase in tissue damping was observed on the 1st day (p = 0.009), and decreases in tissue elastance and tissue damping were only observed on the 28th day after papain administration (p = 0.0012 and p = 0.03, respectively). When analyzing the airway resistance values, no differences were observed at any time.
The Htis, Gtis, and Raw for the papain and saline groups at each time point are represented in Figures 1A, 1B, and 1C. (A) The Htis results are separated into the 1st, 3rd, 15th, 28th, and 40th days. When comparing the groups that received intranasal saline to the papain groups, a significant difference was found on the 28th day (* p = 0.012). (B) Increases in the Gtis values were observed on the 1st (* p = 0.09) and 28th days (* p = 0.03) as compared with the groups that received the intranasal vehicle (saline). (C) No significant differences were found in the Raw values between the experimental groups over time. The values are expressed as means ± SD.
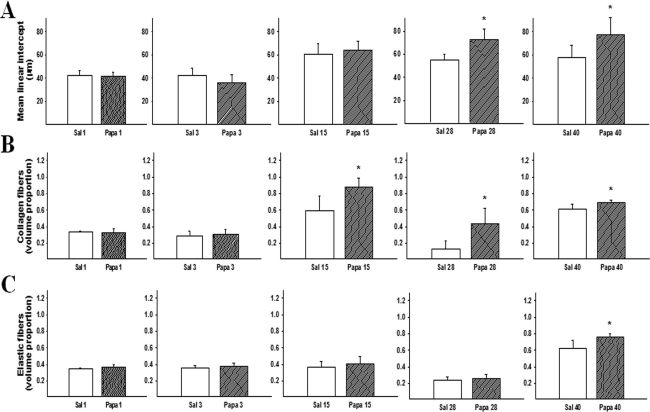
Figure 2A shows the mean Lm values (±SD) for each experimental group. Papain administration resulted in substantial alveolar wall destruction, resulting in an enlargement of the distal air spaces. An increase in Lm was observed on the 28th day and remained until the 40th day (p≤0.001 and p = 0.008, respectively).
(A) Mean linear intercept values measured for the experimental groups. Increases in Lm were observed on the 28th (* p≤0.001) and 40th days (* p = 0.008). The values are expressed as means ± SD. (B) Increases in the volume proportion of collagen fibers in the parenchyma were observed on the 15th (* p = 0.003), 28th (* p = 0.003) and 40th days (* p = 0.012). The values are expressed as means ± SD. (C) The volume proportion of elastic fibers in the parenchyma of the experimental groups was only increased on the 40th day (* p = 0.009). The values are expressed as means ± SD.
The volume proportions of collagen and elastic fibers in the alveolar tissue are shown in Figures 2B and 2C, respectively. Papain administration resulted in a significant increase in the proportion of collagen fibers in the alveolar walls on the 15th (p = 0.003), 28th (p = 0.03), and 40th days (p = 0.012). On the 40th day, the mice that received papain also exhibited an increased proportion of elastic fibers in their alveolar tissue as compared to the mice that received saline (p = 0.009) (Figure 2C).
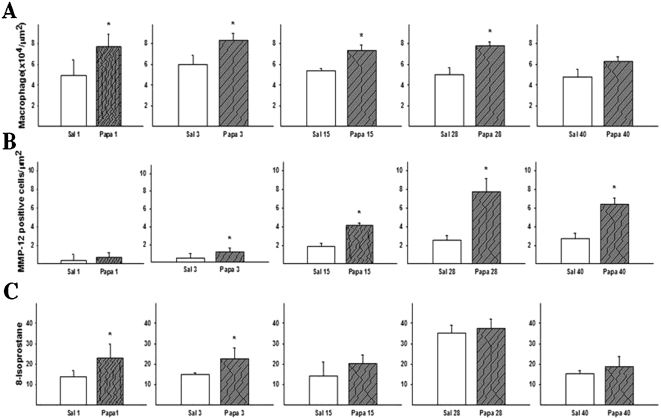
Both the total number of macrophages (Figure 3A) and the number of matrix metalloproteinase 12 (MMP12)-positive cells (Figure 3B) were increased in the mice that received papain. Figure 3A shows the mean (±SD) macrophage numbers for the parenchymas of the different groups. The number of macrophages was increased on the 1st (p = 0.07), 3rd, 15th, 28th, and 40th days (p≤0.001), while the number of MMP12-positive cells was increased from the 3rd day (p = 0.034) until the 40th day (p≤0.001).
(A) The density of macrophages (cells immunostained for MAC-2) was increased on the 1st (* p = 0.007), 3rd, 15th, 28th, and 40th days (p≤0.005 for these groups). (B) MMP12-immunopositive cells in the lung alveolar tissue of the experimental groups, increases were observed on the 3rd (* p = 0.034), 15th, 28th, and 40th days (p≤0.005 for these groups). The values are expressed as means ± SD. (C) Expression of 8-isoprostane measured in the different groups revealed increases on the 1st and 3rd days (* p ≤0.05 for both groups).
Figure 3C shows the mean values (±SD) of 8-isoprostane expression in the parenchyma. Increases in 8-isoprostane expression were only observed in the lung tissue of the mice that received papain on the 1st and 3rd days after administration (p = 0.04).
DISCUSSIONThe current study shows that the parameters evaluated by respiratory mechanics do not always mirror the changes detected by morphometric analysis at various time intervals after papain administration in mice. A significant increase in the mean linear intercept (Lm) associated with decreases in tissue elastance and tissue damping was observed only on the 28th day after papain administration. Additionally, at the same time point, a morphometric evaluation revealed that the number of macrophages, the number of MMP12-expressing cells and the number of collagen fibers in the alveolar parenchyma were increased.
However, on the 40th day, we did not observe differences in the respiratory mechanics parameters; however, all of the morphometric parameters, including the mean linear intercept values, remained increased, indicating the presence of lung emphysema.
When analyzing the volume proportions of elastic and collagen fibers in the parenchyma, an increase in collagen fiber deposition was observed starting on the 15th day, whereas an increase in the number of elastic fibers was only observed on the 40th day. Taken together, these results suggest that extracellular matrix remodeling, particularly that of collagen and elastic fibers, most likely interferes with respiratory mechanics. An increase in elastic force induced by the deposition of collagen and elastic fibers may have the opposite effect on the elastic properties of pulmonary tissue, such as a decrease in alveolar surface area. This effect could explain the lack of a significant difference in tissue elastance when comparing the papain and saline groups at that time.
Although respiratory mechanics measurements have been considered an important strategy for analyzing lung changes in animal models of emphysema, some disparities exist between functional measurements and morphometric analysis. In an earlier study, Foronjy et al.20 found no correlation between the emphysema measured by lung morphometry and that measured by pulmonary compliance in A/J mice exposed to cigarette smoke. They observed that this murine smoke-induced model produced histological emphysema with no changes in pulmonary compliance. In another study, Guerassimov et al.21 analyzed various strains of mice with differential susceptibilities to the development of smoking-induced emphysema and observed that changes in Lm were not always mirrored by changes in lung mechanics after 6 months of smoke exposure. They believed that to alter the mechanical characteristics of a lung, a threshold change in airspace enlargement was necessary. The strains with the greatest change in compliance (elastance) also showed the greatest increase in Lm.
Aside from alveolar destruction, studies have suggested that both the organization and the amount of elastic and collagen fibers determine altered lung function in emphysema. There is evidence for the breakdown and resynthesis of matrix components during the development of experimental emphysema.22,23,24 The manner in which the resynthesis of these matrix components interferes with lung function requires further investigation. Many morphometric and biomechanical studies in humans and animal models have suggested that the presence of collagen in emphysematous lungs is abnormal. In the present study, an increase in collagen deposition was observed on the 15th day, whereas an increase in the number of elastic fibers was only observed on the 40th day.
Four weeks after elastase administration, Kononov et al.25 observed significant remodeling in rat lungs, and this remodeling led to thickened elastin and collagen fibers. Furthermore, Kononov et al. observed that, during stretching, the newly deposited elastin and collagen fibers underwent substantially greater distortion than normal tissues. The threshold for the mechanical failure of collagen, which provides mechanical stability to the normal lung, is reduced during stretching, which suggests that the mechanical forces produced during breathing are capable of causing the failure of the remodeled extracellular matrix and contributing to the progression of emphysema.
The majority of experimental studies of emphysema with protease administration have only analyzed functional and morphometric data after the 21st day of disease induction, and these studies have only found alterations in the tissue elastance and mean linear intercept.3,4,25 Thus far, to our knowledge, no studies have evaluated these parameters at various time points after the induction of emphysema in an animal model to examine how the elastance, mean linear intercept, and remodeling of extracellular matrix fibers change during the development of emphysema.
Animal studies have been important in determining how various mechanisms are involved in the pathophysiology of emphysema.6,26,27 Macrophages are the predominant defense cells, both in normal individuals and COPD patients. MMPs are mainly produced by these cells,27 and an increase in MMP activation in the lungs forms the basis of the protease/anti-protease imbalance hypothesis, which is the prevailing mechanism used to explain the pathogenesis of emphysema. Thus, we decided to verify the number of macrophages and metalloproteinase 12 (MMP12) expression in the lung parenchyma.
Corroborating the results of Shapiro et al.,6 our study shows that a single administration of papain induces an increase in the number of macrophages in the lung parenchyma, beginning on the 1st day and remaining until the 40th day. MMP12 expression is increased on the 3rd day, and this increase is maintained until the 40th day. Such results support the idea that, in this experimental model, the development of emphysema depends on a protease/anti-protease imbalance, starting at the beginning of disease progression.
Oxidative stress is another important mechanism that explains the development of emphysema, and there is evidence for increased lung tissue and systemic oxidative stress in COPD patients.28 Thus, 8-isoprostane expression was also evaluated. Isoprostanes are prostaglandin-like compounds formed by the peroxidation of arachidonic acid and are considered to be accurate oxidative stress markers in vivo, both in humans and experimental animals.29 In a previous study, we found a worsening of emphysema concomitant with an increase in 8-isoprostane expression in mice exposed to air pollution, which contrasted with emphysema in mice that were maintained in a chamber without air pollution.15 In this study, increases in 8-isoprostane expression were observed only on the 1st and 3rd days, suggesting the presence of an oxidant/antioxidant balance starting on the 3rd day. However, this hypothesis needs to be investigated further.
Our study has some limitations. Although cigarette smoking could have been used as a model to induce emphysema (because it more closely mimics the human disease), the development of emphysema in such animal models is lengthy and may only lead to a mild case of the disease.30 Therefore, increases in the numbers of collagen and elastic fibers in these models requires many months of exposure to cigarette smoke. Thus, a protease model was used to induce emphysema. The short time required for disease development can be used to determine which measurements of respiratory mechanics and lung morphometry accurately mirror changes in the lungs and to determine which of these measurements best represents the lung changes in this experimental model.
In conclusion, in this protease-induced model of emphysema by the administration of papain solution, morphometric parameters were found to be more reliable for detecting the presence of emphysema as compared to functional parameters measured by respiratory mechanics. The deposition of collagen and elastic fibers may interfere with the mechanical properties of the respiratory system and could explain the impaired accuracy of the functional measurements.
The authors would like to thank Angela B. dos Santos and Maria Cristina Medeiros for the assistance with immunohistochemical staining.
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Laboratórios de Investigação Médica do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (LIM/HC).
No potential conflict of interest was reported.