The risk factors of bronchiectasis in patients with chronic obstructive pulmonary disease have not yet been established. This systematic review and meta-analysis aimed to investigate and identify potential risk factors for patients with chronic obstructive pulmonary disease accompanied by bronchiectasis. We reviewed eight electronic journal databases from their inception to November 2019 for observational studies with no language restrictions. The Newcastle-Ottawa Scale was applied to evaluate the quality of the literature. Binary variables were pooled using odds ratios and continuous variables using the standardized mean difference with 95% confidence intervals. The confidence of evidence was assessed according to the grading of the recommendations assessment, development, and evaluation method. Eight case-control studies met the inclusion criteria. Tuberculosis history, smoking history, hospitalization stays, admissions in the past year, and duration of symptoms were considered risk factors. In addition, the ratio between the forced expiratory volume in 1s and forced vital capacity, the percentage of forced expiratory volume in 1s, the forced expiratory volume in 1s as a percentage of the predicted value, purulent sputum, purulent mucus sputum, positive sputum culture, Pseudomonas aeruginosa infection, arterial oxygen pressure, daily dyspnea, C-reactive protein, leukocytes, and the percentage of neutrophils were found to be closely related to bronchiectasis. However, these were not considered risk factors. The evidence of all outcomes was judged as “low” or “very low.” Additional prospective studies are required to elucidate the underlying risk factors and identify effective preventive interventions.
Chronic obstructive pulmonary disease (COPD) is one of the main causes of global morbidity and mortality (1) and is characterized by partially reversible, persistent airflow limitation associated with chronic airway inflammation and emphysema (2). COPD is a complex heterogeneous disease (3). The clinical presentation and structural abnormalities of the lung can vary greatly between patients (3). With the increasing application of computed tomography (CT) in the evaluation of patients with COPD, previously unrecognized bronchiectasis is being identified (4). Ko et al. (33) defined the most accepted diagnostic criteria for bronchiectasis. Bronchiectasis is characterized by the irreversible widening of medium to small-sized airways, inflammation, chronic bacterial infection, and destruction of the bronchial walls (5).
Some studies have pointed out that bronchiectasis and COPD may co-exist as an overlap syndrome (6). Bronchiectasis was first defined as a comorbidity of COPD in the Global Chronic Obstructive Pulmonary Disease Initiative 2014 guidelines (7). This change was retained in the 2015 updated version and emphasized the impact of bronchiectasis on the natural history of COPD (6). Multiple studies have shown that bronchiectasis in patients with COPD is associated with increased bronchial inflammation, frequent colonization of potentially pathogenic microorganisms, and severe airflow obstruction (8). Bronchiectasis tends to adversely affect the clinical status of patients with COPD, lower their exercise capacity and quality of life, seriously influence the state of psychology, and cause a poor prognosis (9). Moreover, some cases may be obliged to adopt more efficient and sustained antibiotic therapy, and inhaled corticosteroids may not be suitable for patients with bacterial colonization or recurrent lower respiratory infections (10).
Therefore, identifying the potential risk factors for bronchiectasis in patients with COPD could lead to earlier detection and diagnosis, better guidance for management, more effective treatments, and improvement of health status. However, the risk factors for bronchiectasis in patients with COPD have not been fully confirmed. Several observational studies have investigated them but with small sample sizes. In addition, some contradictory results were found in these studies. For example, Arram and Elrakhawy (11) found that age is a potential risk factor, but the studies by Martínez-García et al. (8) and Yu et al. (12) did not support this result. Thus, this systematic review and meta-analysis aimed to summarize the current evidence of observational studies and then investigate and identify potential risk factors for bronchiectasis in patients with COPD.
MATERIALS AND METHODSResearch registrationThis study was registered on the International Prospective Register of Systematic Reviews (PROSPERO no. CRD 42020171581) and was carried out according to the Meta-analysis Reporting Guide for Observational Research (13).
Search strategyWe conducted a comprehensive retrieval of eight electronic journal databases, including PubMed, Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure, Chinese Biomedical Literature Database, WanFang Database, and Chinese Scientific Journal Database. We reviewed these databases from their inception to November 2019 for observational studies with no restrictions placed on the language of publications. In addition, the bibliographies of identified articles and grey literature were also searched to avoid any omissions. The search strategy of the PubMed database is shown in Table 1, and we adjusted it according to the characteristics of others.
Literature search strategy of the PubMed database
| #1 | “Pulmonary Disease, Chronic Obstructive” [Mesh] |
| #2 | COPD |
| #3 | Chronic Obstructive Pulmonary Disease |
| #4 | COAD |
| #5 | Chronic Obstructive Airway Disease |
| #6 | Chronic Obstructive Lung Disease |
| #7 | Airflow Obstruction, Chronic |
| #8 | Airflow Obstructions, Chronic |
| #9 | Chronic Airflow Obstructions |
| #10 | Chronic Airflow Obstruction |
| #11 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 |
| #12 | “Bronchiectasis” [Mesh] |
| #13 | Bronchiectases |
| #14 | #12 OR #13 |
| #15 | “Risk Factors” [Mesh] |
| #16 | Factor, Risk |
| #17 | Factors, Risk |
| #18 | Risk Factor |
| #19 | Population at Risk |
| #20 | Risk, Population at |
| #21 | Populations at Risk |
| #22 | Risk, Populations at |
| #23 | #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 |
| #24 | #11 AND #14 AND #23 |
COAD, Chronic Obstructive Airways Disease; COPD, Chronic obstructive pulmonary disease.
The inclusion criteria were as follows: 1) eligible observational studies were identified if the risk factors for bronchiectasis in COPD were demonstrated; 2) diagnosis of COPD complies with any version of reliable and accepted guidelines with clear diagnostic criteria and bronchiectasis diagnosed by objective imaging methods such as high-resolution CT (HRCT), CT scan, or chest X-ray (14); 3) studies with all study participants older than 18 years; 4) studies comparing patients with COPD and bronchiectasis in the research group to patients with COPD without bronchiectasis in the control group to identify risk factors; and 5) studies with complete experimental data and results.
The exclusion criteria were as follows: 1) duplicate articles, 2) case report, 3) letters, 4) meeting abstracts, 5) animal experiments, 6) review articles, 7) comment articles, 8) low quality studies, and 9) studies with incomplete data and unclear outcomes.
Literature screeningAll retrieved studies were imported into the Note Express 3.2.0.7350 software (Beijing Aegean Music Technology Co., Ltd.) to delete any duplicates. Two researchers (Zhang XX, Zhang HY) independently screened the titles and abstracts against the established inclusion and exclusion criteria and then downloaded the remaining studies for further screening by reading the full text. If any disagreements occurred, a consensus was reached through discussion or adjudication by a third senior researcher (Pang LJ).
Data extractionThe key characteristics of the included articles were extracted independently by two reviewers (Zhang XX, Zhang HY) using a predefined form. The following data items were collected from each study: the first author, publication year, primary locality of the study, sample size (research group/control group), outcomes, range of age (research group/control group), sex distribution (male/female), diagnostic criteria, and funding. If any important information elements were missing, we attempted to contact the authors for the desired data. If any disagreements occurred during this process, the two reviewers reached a consensus through consultation or adjudication by a third senior investigator (Pang LJ).
Quality assessmentTwo researchers (Zhang XX, Zhang HY) independently and separately applied the Newcastle-Ottawa Scale (NOS) (15) to evaluate the quality of the included literature, which contains three aspects: selection, comparability, and exposure/outcome. Those studies with a score of 5 or more were classified as high quality, while those with a score lower than 5 were classified as low quality (16). To ensure the reliability of the results, low quality literature were not be included in the meta-analysis. Any disagreement during this period was discussed with a third senior researcher (Lv XD). The AMSTAR 2 checklist was used to evaluate the methodological quality of this meta-analysis by two researchers independently (Zhang XX, Zhang HY). This checklist includes 16 criteria. The methodological quality score ranged from 0 to 16. Scores of 15-16, 12-14, 9-11, 6-8, and 3-5 items were evaluated as excellent, very good, good, acceptable, and deficient, respectively (17). Disagreements were resolved by consensus with a third investigator (Lv XD).
Statistical analysisThe Stata13.1 software (Stata-Corp LP, College Station TX77845) was used for the meta-analysis. The Q-test and I2 values were applied to measure the inter-study heterogeneity. When the p-value of Q-test>0.1 and I2<50%, a fixed-effects model was applied; otherwise, a random-effects model was used. Binary variables were expressed using the odds ratio with 95% confidence interval (CI) and continuous variables by the standardized mean difference with 95% CI. Forest plots were created using GraphPad Prism version 7.00 software. A subgroup analysis was used to explore the potential confounding factors for significant heterogeneity, such as age, country, literature quality, and publication year. A sensitivity analysis was carried out by removing individual studies to measure the robustness of the results. Egger and Peters tests (18) were performed to provide quantitative evidence of any publication bias (n>10).
The grading of recommendations assessment, development, and evaluation (GRADE) algorithm (19) was used to assign quality levels to the meta-analysis evidence. The overall confidence could be judged as “high,” “moderate,” “low,” or “very low.”
RESULTSLiterature selectionA total of 1034 studies were initially identified. Of these, 196 were excluded as they were duplicate studies, and 166 were excluded following a review of the title or abstract. A total of 672 studies remained for full text review. Of these, 664 were excluded as they did not meet the eligibility criteria. Finally, the eight remaining articles (7,8,11,12,20–23) were included in this meta-analysis, including four in Chinese and four in English. All of these were case-control studies. A flowchart of the literature screening and selection process is shown in Figure 1.
Flowchart of the search strategy and inclusion of the studies according to the preferred reporting items for systematic reviews and meta-analyses statement. CBM: Chinese Biomedical Literature Database; CNKI: China National Knowledge Infrastructure; VIP: VIP Database for Chinese Technical Periodicals; WOS: Web of Science.
Two reviewers independently summarized the characteristics of the included studies according to the data extraction process. A total of 1669 patients were involved, which included 692 in the research group and 977 in the control group. The primary localities of the studies were distributed in three countries, six provinces, and municipalities. The median NOS score of the included studies was 6, with a range from 5 to 7, indicating that these studies were of high quality. The key characteristics of the included studies are presented in Table 2. As evaluated by the AMSTAR2 tool, this meta-analysis scored “very good.” Only questions 7 and 9 were evaluated as “No,” and the rest were evaluated as “Yes.”
Summary of the study design and study characteristics.
| Study | Publication year | Locality | Sample size (T/C) | Quality assessment (NOS score) | Research factors | Age (T/C) | Sex (Male/Female) | Diagnostic criteria | Funder |
|---|---|---|---|---|---|---|---|---|---|
| Qin (20) | 2018 | Jilin | 198/282 | 6 | 1.4.6.8.9.10.11.16.17.18.19.20.22.24.25.26.30.31.32.33.34.35 | 69.88±9.72/69.95±9.73 | 257/223 | b (2017 version) HRCT | NA |
| Liu et al. (21) | 2019 | Anhui | 57/96 | 5 | 1.2.3.4.10.11.13.16.17.18.24.25.26.28.30.31.32.33.34 | 69.57±9.64/66.34±9.52 | 94/59 | b HRCT | Anhui Provincial Health Department (13ZC024) |
| Pan et al. (22) | 2019 | Shanghai | 135/217 | 6 | 1.2.3.4.6.8.9.10.11.16.17.18.19.20.22.24.25.31.32.33.34.35 | 62.81±10.42/64.37±9.15 | 184/168 | a (2013 revision) HRCT | NA |
| Zhao (23) | 2015 | Hebei | 86/114 | 5 | 5.6.7.10.11.12.13.14.15.16.17.18.19.21.27.29 | 67.79±9.27/69.48±10.02 | 173/27 | a (2007 revision) b (2011 version) HRCT | NA |
| Jin et al. (7) | 2016 | Beijing | 87/103 | 6 | 1.4.5.8.9.10.11.30.35.36.37 | 77.0/78.9 | 121/69 | clinical diagnosis HRCT | National Natural Science Foundation of China (81170039, 81470239); Beijing Talent Training Foundation (No. 2009D003003000002) |
| Martínez-García et al. (8) | 2011 | Spain | 53/39 | 7 | 1.4.5.6.10.11.12.13.16.28.37 | 72.6/69.1 | 91/1 | clinical diagnosis HRCT | A public grant from the Sociedad Valenciana de Neumología |
| Yu et al. (12) | 2019 | Tianjin | 43/90 | 6 | 1.5.6.8.10.11.16.17.20.21.23.26.27.30.33.35.36 | 71.02±8.47/69.78±8.24 | 83/50 | clinical diagnosis HRCT | None |
| Arram et al. (11) | 2012 | Egypt | 33/36 | 7 | 5.6.7.10.11.12.13.14.15.16.17.19.23.27.29 | 63.79±5.41/56.50±5.56 | 4/65 | b (2009 version) HRCT | NA |
1. Tuberculosis history 2. Hypertension history 3. Diabetes history 4. Smoking history 5. Smoking index (pack/year) 6. FEV1/FVC 7. FEV1% 8. FEV1%pred 9. BMI 10. Age 11. Female 12. Mucous sputum 13. Purulent sputum 14. Purulent mucus sputum, 15. Positive sputum culture, 16. Pseudomonas aeruginosa infection 17. Klebsiella pneumoniae infection, 18. Escherichia coli infection 19. Streptococcus pneumoniae infection 20. Acinetobacter baumannii infection 21. Stenotrophomonas maltophilia infection 22. Enterobacter cloacae infection 23. Haemophilus infection, 24. PaCO2 25. PaO2 26. Hospital stays 27. Admission within the past year 28. Duration of symptoms 29. Daily dyspnea 30. CRP 31. ESR 32. Hb 33. WBC 34. N% 35. FIB 36. Albumin 37. Anticholinergic therapy.
a. Guidelines for the diagnosis and treatment of chronic obstructive pulmonary disease.
b. The Global Initiative for Chronic Obstructive Lung Disease.
BMI, body mass index; C, control group; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FEV1/FVC, the ratio between forced expiratory volume in 1s and forced vital capacity; FEV1%, the percentage of FEV1;. FEV1%pred, FEV1 as a percentage of the predicted value; FIB, plasma fibrinogen; Hb, hemoglobin; HRCT, High Resolution Computed Tomography; N%, percentage of neutrophils; NA, unclear or not mentioned; None, no funding; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen pressure; WBC, leukocyte; T, trial group.
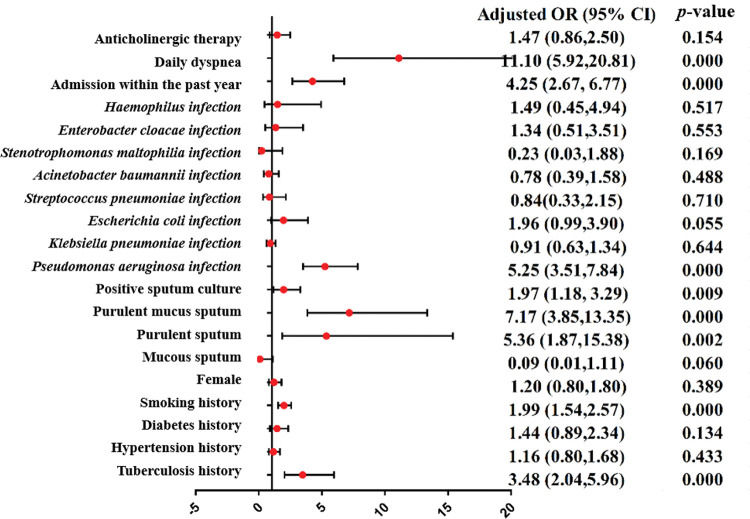
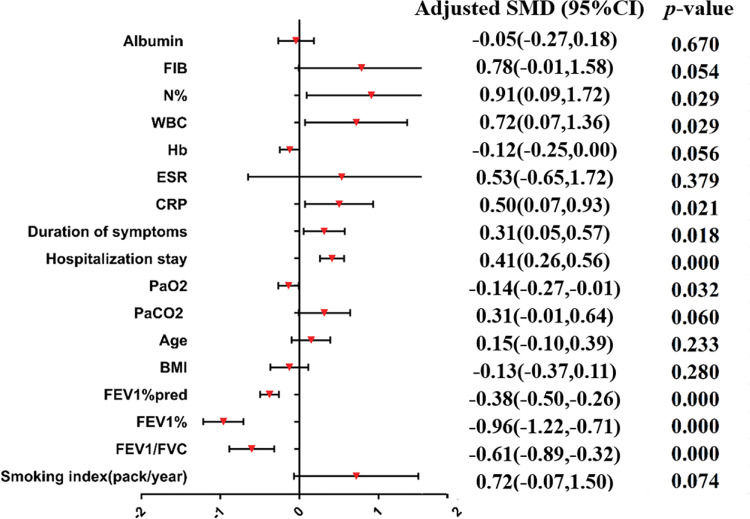
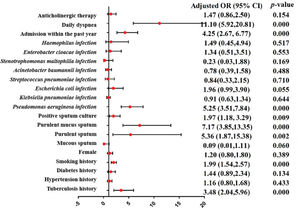
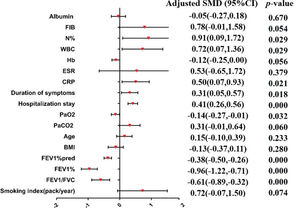
A meta-analysis was applied to the indicators of the eight included studies. The results show that the indicators were statistically significant between the research group and control group (p<0.05), including tuberculosis history, smoking history, the ratio between forced expiratory volume in 1s and forced vital capacity (FEV1/FVC), the percentage of FEV1 (FEV1%), the FEV1as a percentage of the predicted value (FEV1%pred), purulent sputum, purulent mucus sputum, positive sputum culture, Pseudomonas aeruginosa infection, arterial oxygen pressure (PaO2), hospital stay, admission within the past year, duration of symptoms, daily dyspnea, C-reactive protein (CRP), leukocytes (WBC), and the percentage of neutrophils (N%). The results of the heterogeneity test, model, effect size, 95% CI, and p-values are shown in Table 3. The forest plots of the two types of variable indexes are described in Figures 2 and 3.
Results of the meta-analysis of various indicators.
| Heterogeneity test | Sensitivity analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Number | Q-test | I2 (%) | Effect model | Effect selection | Effect size and 95%CI | p-value | I2 (%) | p-value | excluded |
| Tuberculosis history | 6 | 0.023 | 61.7% | Random | OR | 3.48 (2.04,5.96) | 0.000 | |||
| Hypertension history | 2 | 0.844 | 0.0% | Fixed | OR | 1.16 (0.80,1.68) | 0.433 | |||
| Diabetes history | 2 | 0.243 | 26.7% | Fixed | OR | 1.44 (0.89,2.34) | 0.134 | |||
| Smoking history | 5 | 0.141 | 42.0% | Fixed | OR | 1.99 (1.54,2.57) | 0.000 | |||
| Smoking index (pack/year) | 5 | 0.000 | 95.5% | Random | SMD | 0.72 (−0.07,1.50) | 0.074 | 95.0% | 0.025 | Yu et al. (12) |
| FEV1/FVC | 6 | 0.000 | 81.7% | Random | SMD | −0.61 (−0.89,-0.32) | 0.000 | |||
| FEV1% | 2 | 0.198 | 39.7% | Fixed | SMD | −0.96 (−1.22,-0.71) | 0.000 | |||
| FEV1%pred | 4 | 0.977 | 0.0% | Fixed | SMD | −0.38 (−0.50,-0.26) | 0.000 | |||
| BMI | 3 | 0.031 | 71.1% | Random | SMD | −0.13 (−0.37,0.11) | 0.280 | 0.0% | 0.003 | Pan et al. (22) |
| Age | 8 | 0.000 | 81.7% | Random | SMD | 0.15 (−0.10,0.39) | 0.233 | |||
| Female sex | 8 | 0.017 | 59.0% | Random | OR | 1.20 (0.80,1.80) | 0.389 | |||
| Mucous sputum | 3 | 0.000 | 90.9% | Random | OR | 0.09 (0.01,1.11) | 0.060 | 82.4% | 0.030 | Martínez-García et al. (8) |
| Purulent sputum | 4 | 0.039 | 64.1% | Random | OR | 5.36 (1.87,15.38) | 0.002 | 68.4%72.6% | 0.1200.073 | Liu et al. (21)/Zhao (23) |
| Purulent mucus sputum | 2 | 0.751 | 0.0% | Fixed | OR | 7.17 (3.85,13.35) | 0.000 | |||
| Positive sputum culture | 2 | 0.638 | 0.0% | Fixed | OR | 1.97 (1.18, 3.29) | 0.009 | |||
| Pseudomonas aeruginosa infection | 7 | 0.916 | 0.0% | Fixed | OR | 5.25 (3.51,7.84) | 0.000 | |||
| Klebsiella pneumoniae infection | 6 | 0.096 | 46.6% | Fixed | OR | 0.91 (0.63,1.34) | 0.644 | |||
| Escherichia coli infection | 4 | 0.747 | 0.0% | Fixed | OR | 1.96 (0.99,3.90) | 0.055 | |||
| Streptococcus pneumoniae infection | 4 | 0.860 | 0.0% | Fixed | OR | 0.84 (0.33,2.15) | 0.710 | |||
| Acinetobacter baumannii infection | 3 | 0.687 | 0.0% | Fixed | OR | 0.78 (0.39,1.58) | 0.488 | |||
| Stenotrophomonas maltophilia infection | 2 | 0.836 | 0.0% | Fixed | OR | 0.23 (0.03,1.88) | 0.169 | |||
| Enterobacter cloacae infection | 2 | 0.865 | 0.0% | Fixed | OR | 1.34 (0.51,3.51) | 0.553 | |||
| Haemophilus infection | 2 | 0.324 | 0.0% | Fixed | OR | 1.49 (0.45,4.94) | 0.517 | |||
| PaCO2 | 3 | 0.003 | 82.5% | Random | SMD | 0.31 (−0.01,0.64) | 0.060 | 0.0% | 0.064 | Liu et al. (21) |
| PaO2 | 3 | 0.518 | 0.0% | Fixed | SMD | −0.14 (−0.27,-0.01) | 0.032 | 23.9%0.0% | 0.0850.326 | Pan et al. (22)/Qin (20) |
| Hospital stay | 3 | 0.749 | 0.0% | Fixed | SMD | 0.41 (0.26,0.56) | 0.000 | |||
| Admission within the past year | 3 | 0.250 | 27.9% | Fixed | OR | 4.25 (2.67, 6.77) | 0.000 | |||
| Duration of symptoms | 2 | 0.554 | 0.0% | Fixed | SMD | 0.31 (0.05,0.57) | 0.018 | |||
| Daily dyspnea | 2 | 0.343 | 0.0% | Fixed | OR | 11.10 (5.92,20.81) | 0.000 | |||
| CRP | 4 | 0.000 | 89.0% | Random | SMD | 0.50 (0.07,0.93) | 0.021 | 91.1%0.0% | 0.0600.001 | Jin et al. (7)/Qin (20) |
| ESR | 3 | 0.000 | 98.5% | Random | SMD | 0.53 (−0.65,1.72) | 0.379 | 0.0% | 0.000 | Liu (21) |
| Hb | 3 | 0.138 | 49.5% | Fixed | SMD | −0.12 (−0.25,0.00) | 0.056 | 0.0% | 0.007 | Qin (20) |
| WBC | 4 | 0.000 | 95.7% | Random | SMD | 0.72 (0.07,1.36) | 0.029 | 0.0% | 0.294 | Qin (20)/Pan et al. (22) |
| N% | 3 | 0.000 | 96.9% | Random | SMD | 0.91 (0.09,1.72) | 0.029 | 95.9%98.4% | 0.2800.294 | Qin (20)/Pan et al. (22) |
| FIB | 4 | 0.000 | 97.3% | Random | SMD | 0.78 (−0.01,1.58) | 0.054 | 80.3%97.6% | 0.0250.030 | Qin (20)/Yu et al. (12) |
| Albumin | 2 | 0.320 | 0.0% | Fixed | SMD | −0.05 (−0.27,0.18) | 0.670 | |||
| Anticholinergic therapy | 2 | 0.536 | 0.0% | Fixed | OR | 1.47 (0.86,2.50) | 0.154 | |||
The blank lines in the sensitivity analysis columns indicate that the results were stable.
BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FEV1/FVC, the ratio between forced expiratory volume in 1 s and forced vital capacity; FEV1%, the percentage of FEV1;. FEV1%pred, FEV1 as a percentage of the predicted value; FIB, plasma fibrinogen; GRADE, grading of recommendations assessment, development, and evaluation; Hb, hemoglobin; N%, percentage of neutrophils; OR, odds ratio; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen pressure; SMD, standardized mean difference; WBC, leukocytes
Forest-plot of the continuous variable index (SMD). BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FEV1/FVC, the ratio between forced expiratory volume in 1s and forced vital capacity; FEV1%, the percentage of FEV1;. FEV1%pred, FEV1 as a percentage of the predicted value; FIB, plasma fibrinogen; Hb, hemoglobin; N%, percentage of neutrophils; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen pressure; SMD, standardized mean difference; WBC, leukocytes.
Reversed results of certain factors existed according to the sensitivity analysis. The lower heterogeneity and stable results emerged after excluding data on arterial carbon dioxide partial pressure (PaCO2) and CRP. The specific results are listed in Table 3. The results of the remaining factors were unchanged after the sensitivity analysis, suggesting that the results should be more stable.
A subgroup analysis was used to explore the sources of heterogeneity for the indicators. For the factor of age, a subgroup analysis was conducted with two groups according to the country (Asian/non-Asian). There was no change in the Asian group but statistical significance in the non-Asian group. The factor of purulent sputum was analyzed in the subgroup analysis according to the country (Asian/non-Asian). The results showed no change in the non-Asian group. In contrast, there was no statistical significance and lower heterogeneity in the Asian group. Therefore, the country where the study was conducted may be a confounding factor and source of heterogeneity, and more research will be needed in the future.
Sensitivity analysis and GRADE evaluationThe robustness of the results in the sensitivity analysis was good, except for smoking index, body mass index (BMI), mucous sputum, purulent sputum, PaCO2, PaO2, CRP, erythrocyte sedimentation rate (ESR), hemoglobin (Hb), plasma fibrinogen (FIB), WBC, and N%. The sensitivity analysis indicated heterogeneity in the strengths of the association due to the most common biases in observational studies. The GRADE evidence of all outcomes was judged as “low” or “very low.” The results are shown in Tables 4 and 5.
GRADE evidence profile.
| Quality assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Effect | ||||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Bronchiectasis | No bronchiectasis | Relative (95%CI) | Absolute (95%CI) | Quality |
| Tuberculosis history | |||||||||||
| 6 | Observational study | not serious | seriousa | not serious | seriouso | not found | 176/573 | 103/827 | RR 2.49 (1.65 to 3.75) | 96 per 1000 | ⊕○○○ |
| Smoking history | |||||||||||
| 5 | Observational study | not serious | not serious | not serious | not serious | not found | 367/530 | 393/737 | RR 1.25 (1.15 to 1.35) | 635 per 1000 | ⊕⊕○○ |
| Pseudomonas aeruginosa infection | |||||||||||
| 7 | Observational study | not serious | not serious | not serious | seriouso | not found | 107/605 | 35/874 | RR 4.36 (3.04 to 6.27) | 28 per 1000 | ⊕○○○ |
| Purulent sputum | |||||||||||
| 4 | Observational study | not serious | seriousb | not serious | seriouso | not found | 83/229 | 35/285 | RR 3.09 (1.68 to 5.69) | 70 per 1000 | ⊕○○○ |
| Purulent mucus sputum | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | serious o | not found | 57/119 | 17/150 | RR 4.18 (2.57 to 6.79) | 122 per 1000 | ⊕○○○ |
| Positive sputum culture | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso | not found | 51/119 | 41/150 | RR 1.56 (1.11 to 2.17) | 284 per 1000 | ⊕○○○ |
| Admission within the past year | |||||||||||
| 3 | Observational study | not serious | not serious | not serious | seriouso | not found | 110/162 | 85/240 | RR 1.82 (1.51 to 2.20) | 222 per 1000 | ⊕○○○ |
| Daily dyspnea | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso | not found | 101/119 | 54/150 | RR 2.40 (1.91 to 3.01) | 294 per 1000 | ⊕○○○ |
| Hypertension history | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 76/192 | 113/313 | RR 1.10 (0.87 to 1.38) | 362 per 1000 | ⊕○○○ |
| Diabetes history | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 40/192 | 50/313 | RR 1.32 (0.92 to 1.90) | 208 per 1000 | ⊕○○○ |
| Female | |||||||||||
| 8 | Observational study | not serious | seriousc | not serious | serious p | not found | 259/692 | 374/977 | RR 1.09 (0.91 to 1.31) | 328 per 1000 | ⊕○○○ |
| Mucous sputum | |||||||||||
| 3 | Observational study | not serious | seriousd | not serious | seriouso,p | not found | 69/172 | 156/189 | RR 0.29 (0.05 to 1.66) | 546 per 1000 | ⊕○○○ |
| Klebsiella pneumoniae infection | |||||||||||
| 6 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 47/552 | 78/835 | RR 0.92 (0.66 to 1.30) | 49 per 1000 | ⊕○○○ |
| Escherichia coli infection | |||||||||||
| 4 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 19/476 | 15/709 | RR 1.92 (0.99 to 3.75) | 21 per 1000 | ⊕○○○ |
| Streptococcus pneumoniae infection | |||||||||||
| 4 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 7/452 | 11/649 | RR 0.84 (0.35 to 2.05) | 10 per 1000 | ⊕○○○ |
| Acinetobacter baumannii infection | |||||||||||
| 3 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 12/376 | 24/589 | RR 0.79 (0.40 to 1.55) | 41 per 1000 | ⊕○○○ |
| Stenotrophomonas maltophilia infection | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 0/129 | 6/204 | RR 0.23 (0.03 to 1.86) | 30 per 1000 | ⊕○○○ |
| Enterobacter cloacae infection | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 8/333 | 9/499 | RR 1.33 (0.52 to 3.42) | 18 per 1000 | ⊕○○○ |
| Haemophilus infection | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 6/76 | 5/126 | RR 1.42 (0.50 to 3.98) | 69 per 1000 | ⊕○○○ |
| Anticholinergic therapy | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriouso,p | not found | 107/140 | 96/142 | RR 1.11 (0.96 to 1.28) | 713 per 1000 | ⊕○○○ |
| FEV1/FVC | |||||||||||
| 6 | Observational study | not serious | seriouse | not serious | not serious | not found | 548 | 778 | − | SMD-0.61 (−0.89 to −0.32) | ⊕○○○ |
| FEV1% | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriousq | not found | 119 | 150 | − | SMD-0.96 (−1.22 to −0.71) | ⊕○○○ |
| FEV1%pred | |||||||||||
| 4 | Observational study | not serious | not serious | not serious | not serious | not found | 463 | 692 | − | SMD-0.38 (−0.50 to −0.26) | ⊕⊕○○ |
| PaO2 | |||||||||||
| 3 | Observational study | not serious | not serious | not serious | not serious | not found | 390 | 595 | − | SMD-0.14 (−0.27 to −0.01) | ⊕⊕○○ |
| Hospital stay | |||||||||||
| 3 | Observational study | not serious | not serious | not serious | not serious | not found | 298 | 468 | − | SMD 0.41 (0.26 to 0.56) | ⊕⊕○○ |
| Duration of symptoms | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriousq | not found | 110 | 135 | − | SMD 0.31 (0.05 to 0.57) | ⊕○○○ |
| CRP | |||||||||||
| 4 | Observational study | not serious | serious f | not serious | not serious | not found | 385 | 571 | − | SMD 0.50 (0.07 to 0.93) | ⊕○○○ |
| WBC | |||||||||||
| 4 | Observational study | not serious | seriousg | not serious | not serious | not found | 433 | 685 | − | SMD 0.72 (0.07 to 1.36) | ⊕○○○ |
| N% | |||||||||||
| 3 | Observational study | not serious | serioush | not serious | not serious | not found | 816 | 1116 | − | SMD 0.91 (0.09 to 1.72) | ⊕○○○ |
| Smoking index (pack/year) | |||||||||||
| 5 | Observational study | not serious | seriousi | not serious | not serious | not found | 302 | 382 | − | SMD 0.72 (−0.07 to 1.50) | ⊕○○○ |
| BMI | |||||||||||
| 3 | Observational study | not serious | serious j | not serious | not serious | not found | 420 | 602 | − | SMD −0.13 (−0.37 to 0.11) | ⊕○○○ |
| Age | |||||||||||
| 8 | Observational study | not serious | seriousk | not serious | not serious | not found | 692 | 977 | − | SMD 0.15 (−0.10 to 0.39) | ⊕○○○ |
| PaCO2 | |||||||||||
| 3 | Observational study | not serious | seriousl | not serious | not serious | not found | 390 | 595 | − | SMD 0.31 (−0.01 to 0.64) | ⊕○○○ |
| ESR | |||||||||||
| 3 | Observational study | not serious | seriousm | not serious | not serious | not found | 390 | 595 | − | SMD 0.53 (−0.65 to 1.72) | ⊕○○○ |
| Hb | |||||||||||
| 3 | Observational study | not serious | not serious | not serious | not serious | not found | 390 | 595 | − | SMD −0.12 (−0.25 to 0.00) | ⊕⊕○○ |
| FIB | |||||||||||
| 4 | Observational study | not serious | seriousn | not serious | not serious | not found | 463 | 692 | − | SMD 0.78 (−0.01 to 1.58) | ⊕○○○ |
| Albumin | |||||||||||
| 2 | Observational study | not serious | not serious | not serious | seriousq | not found | 130 | 193 | − | SMD −0.05 (−0.27 to 0.18) | ⊕○○○ |
BMI, body mass index; CI, credible interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FEV1/FVC, the ratio between forced expiratory volume in 1s and forced vital capacity; FEV1%, the percentage of FEV1;. FEV1%pred, FEV1 as a percentage of the predicted value; FIB, plasma fibrinogen; GRADE, grading of recommendations assessment, development, and evaluation; Hb, hemoglobin; N%, percentage of neutrophils; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen pressure; RR, risk ratio; SMD, standardized mean difference; WBC, leukocytes.
GRADE Working Group grades of evidence High quality(⊕⊕⊕⊕): Further research is unlikely to change our confidence in the estimate of effect. Moderate quality(⊕⊕⊕○): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality(⊕⊕○○): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality(⊕○○○): We are very uncertain about the estimate.
a. I2=61.7%; b. I2=64.1%; c. I2=59.0%; d. I2=90.9%; e. I2=81.7%; f. I2=89.0%; g. I2=95.7%; h. I2=96.9%; i. I2=95.5%; j. I2=71.1%; k. I2=81.7%; l. I2=82.5%; m. I2=98.5%; n. I2=97.3%; o. The total sample size was less than the optimal information size, p. The 95%CI of the pooled estimate included one or no effect; q. The total sample size was less than 400.
GRADE summary of findings.
| Outcome | Anticipated absolute effects (95%CI) | Relative effect (95%CI) | No. of participants (Studies) | Quality | Comments | |
|---|---|---|---|---|---|---|
| Risk with no bronchiectasis | Risk with bronchiectasis | |||||
| Tuberculosis history | 96 per 1000 | 239 per 1000 (158 to 360) | RR 2.49 (1.65 to 3.75) | 1400 (6) | ⊕○○○ | |
| Smoking history | 635 per 1000 | 794 per 1000 (730 to 857) | RR 1.25 (1.15 to 1.35) | 1267 (5) | ⊕⊕○○ | |
| Pseudomonas aeruginosa infection | 28 per 1000 | 122 per 1000 (85 to 176) | RR 4.36 (3.04 to 6.27) | 1479 (7) | ⊕○○○ | |
| Purulent sputum | 70 per 1000 | 216 per 1000 (117 to 398) | RR 3.09 (1.68 to 5.69) | 514 (4) | ⊕○○○ | |
| Purulent mucus sputum | 122 per 1000 | 510 per 1000 (314 to 828) | RR 4.18 (2.57 to 6.79) | 269 (2) | ⊕○○○ | |
| Positive sputum culture | 284 per 1000 | 443 per 1000 (315 to 616) | RR 1.56 (1.11 to 2.17) | 269 (2) | ⊕○○○ | |
| Admission within the past year | 222 per 1000 | 404 per 1000 (335 to 488) | RR 1.82 (1.51 to 2.20) | 402 (3) | ⊕○○○ | |
| Daily dyspnea | 294 per 1000 | 706 per 1000 (562 to 885) | RR 2.40 (1.91 to 3.01) | 269 (2) | ⊕○○○ | |
| Hypertension history | 362 per 1000 | 398 per 1000 (315 to 500) | RR 1.10 (0.87 to 1.38) | 505 (2) | ⊕○○○ | |
| Diabetes history | 208 per 1000 | 275 per 1000 (191 to 395) | RR 1.32 (0.92 to 1.90) | 505 (2) | ⊕○○○ | |
| Female | 328 per 1000 | 358 per 1000 (298 to 430) | RR 1.09 (0.91 to 1.31) | 1669 (8) | ⊕○○○ | |
| Mucous sputum | 546 per 1000 | 158 per 1000 (27 to 906) | RR 0.29 (0.05 to 1.66) | 361 (3) | ⊕○○○ | |
| Klebsiella pneumoniae infection | 49 per 1000 | 45 per 1000 (32 to 64) | RR 0.92 (0.66 to 1.30) | 1387 (6) | ⊕○○○ | |
| Escherichia coli infection | 21 per 1000 | 40 per 1000 (20 to 79) | RR 1.92 (0.99 to 3.75) | 1185 (4) | ⊕○○○ | |
| Streptococcus pneumoniae infection | 10 per 1000 | 8 per 1000 (4 to 21) | RR 0.84 (0.35 to 2.05) | 1101 (4) | ⊕○○○ | |
| Acinetobacter baumannii infection | 41 per 1000 | 32 per 1000 (16 to 64) | RR 0.79 (0.40 to 1.55) | 965 (3) | ⊕○○○ | |
| Stenotrophomonas maltophilia infection | 30 per 1000 | 7 per 1000 (1 to 56) | RR 0.23 (0.03 to 1.86) | 333 (2) | ⊕○○○ | |
| Enterobacter cloacae infection | 18 per 1000 | 24 per 1000 (9 to 62) | RR 1.33 (0.52 to 3.42) | 832 (2) | ⊕○○○ | |
| Haemophilus infection | 69 per 1000 | 98 per 1000 (35 to 275) | RR 1.42 (0.50 to 3.98) | 202 (2) | ⊕○○○ | |
| Anticholinergic therapy | 713 per 1000 | 791 per 1000 (684 to 913) | RR 1.11 (0.96 to 1.28) | 282 (2) | ⊕○○○ | |
| FEV1/FVC | The mean FEV1/FVC in the control group was 0 | The mean FEV1/FVC in the trial group was 0.61 standard deviations lower (0.89 lower to 0.32 lower) | − | 1326 (6) | ⊕○○○ | |
| FEV1% | The mean FEV1% in the control group was 0 | The mean FEV1% in the trial group was 0.96 standard deviations lower (1.22 lower to 0.71 lower) | − | 269 (2) | ⊕○○○ | |
| FEV1%pred | The mean FEV1%pred in the control group was 0 | The mean FEV1%pred in the trial group was 0.38 standard deviations lower (0.50 lower to 0.26 lower) | − | 1155 (4) | ⊕⊕○○ | |
| PaO2 | The mean PaO2 in the control group was 0 | The mean PaO2 in the trial group was 0.14 standard deviations lower (0.27 lower to 0.01 lower) | − | 985 (3) | ⊕⊕○○ | |
| Hospital stay | The mean hospital stay in the control group was 0 | The mean hospital stay in the trial group was 0.41 standard deviations higher (0.26 lower to 0.56 higher) | − | 766 (3) | ⊕⊕○○ | |
| Duration of symptoms | The mean duration of symptoms in the control group was 0 | The mean duration of symptoms in the trial group was 0.31 standard deviations higher (0.05 lower to 0.57 higher) | − | 245 (2) | ⊕○○○ | |
| CRP | The mean CRP in the control group was 0 | The mean CRP in the trial group was 0.50 standard deviations higher (0.07 lower to 0.93 higher) | − | 956 (4) | ⊕○○○ | |
| WBC | The mean WBC in the control group was 0 | The mean WBC in the trial group was 0.72 standard deviations higher (0.07 lower to 1.36 higher) | − | 1118 (4) | ⊕○○○ | |
| N% | The mean N% in the control group was 0 | The mean N% in the trial group was 0.91 standard deviations higher (0.09 lower to 1.72 higher) | − | 1932 (3) | ⊕○○○ | |
| Smoking index (pack/year) | The mean smoking index in the control group was 0 | The mean smoking index in the trial group was 0.72 standard deviations higher (0.07 lower to 1.50 higher) | − | 684 (5) | ⊕○○○ | |
| BMI | The mean BMI in the control group was 0 | The mean BMI in the trial group was 0.13 standard deviations lower (0.37 lower to 0.11 higher) | − | 1022 (3) | ⊕○○○ | |
| Age | The mean age in the control group was 0 | The mean age in the trial group was 0.15 standard deviations higher (0.10 lower to 0.39 higher) | − | 1669 (8) | ⊕○○○ | |
| PaCO2 | The mean PaCO2 in the control group was 0 | The mean PaCO2 in the trial group was 0.31 standard deviations higher (0.01 lower to 0.64 higher) | − | 985 (3) | ⊕○○○ | |
| ESR | The mean ESR in the control group was 0 | The mean ESR in the trial group was 0.53 standard deviations higher (0.65 lower to 1.72 higher) | − | 985 (3) | ⊕○○○ | |
| Hb | The mean Hb in the control group was 0 | The mean Hb in the trial group was 0.12 standard deviations lower (0.25 lower to 0.00 higher) | − | 985 (3) | ⊕⊕○○ | |
| FIB | The mean FIB in the control group was 0 | The mean FIB in the trial group was 0.78 standard deviations higher (0.01 lower to 1.58 higher) | − | 1155 (4) | ⊕○○○ | |
| Albumin | The mean albumin in the control group was 0 | The mean albumin in the trial group was 0.05 standard deviations lower (0.27 lower to 0.18 higher) | − | 323 (2) | ⊕○○○ | |
BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FEV1/FVC, the ratio between forced expiratory volume in 1s and forced vital capacity; FEV1%, the percentage of FEV1;. FEV1%pred, FEV1 as a percentage of the predicted value; FIB, plasma fibrinogen; GRADE, grading of recommendations assessment, development, and evaluation; Hb, hemoglobin; N%, percentage of neutrophils; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen pressure; RR, risk ratio; WBC, leukocytes.
The prevention of bronchiectasis is important in the treatment of patients with COPD. However, until now, the risk factors of bronchiectasis have not been confirmed. This study demonstrated a clear relationship between patients with COPD and bronchiectasis and certain risk factors, helping us to better understand the disease. Several case-control studies included in this article suggested some risk factors for bronchiectasis in patients with COPD (Table 3). The results showed that the risk factors for bronchiectasis in COPD might include tuberculosis history, smoking history, hospitalization stay, admission within the past year, and duration of symptoms. In addition, FEV1/FVC, FEV1%, FEV1%pred, purulent sputum, purulent mucus sputum, positive sputum culture, Pseudomonas aeruginosa infection, PaO2, daily dyspnea, CRP, WBC, and N% were clinical symptoms of bronchiectasis. They were closely related to bronchiectasis in COPD but were not regarded as risk factors. The lung lumens and parenchyma of patients with COPD with a history of tuberculosis were destroyed, which could lead to prolonged airway inflammation duration and acceleration of lung injury and severe airflow obstruction, thus increasing the incidence of bronchiectasis (22). Therefore, patients with a history of tuberculosis should also undergo regular follow-up, although the disease has been cured. Smoking tended to affect lung function. Therefore, it is necessary for patients with COPD to quit smoking. The lung function of patients with COPD was directly impaired due to irreversible airflow limitation. The lung function indicators progressively decreased, which negatively correlated with the number of damaged lung lobes (25). This tends to induce COPD deterioration, and the second most prevalent cause of bronchiectasis was COPD (26).
Consequently, patients with COPD need to monitor lung function indicators regularly to avoid further deterioration. COPD usually has recurrent attacks and is difficult to cure. If patients cough up purulent sputum, this can lead to a considerably greater magnitude of airway dysbiosis (27). However, purulent sputum is not regarded as a risk factor for bronchiectasis in COPD. Bacterial colonization of the airway was the main inducer of airway inflammation in bronchiectasis (24). Positive sputum culture in patients with COPD demonstrated an imbalance of autoimmune function, which increased the host's predisposition to diseases. The most common pathogenic microorganism, such as Pseudomonas aeruginosa (28), causes chronic inflammation and lung injury aggravations and increases the incidence of bronchiectasis. However, positive sputum culture and Pseudomonas aeruginosa infection were not considered risk factors for bronchiectasis in patients with COPD because these symptoms were present in bronchiectasis. The overall result of PaO2 described in the literature was significant, but the results were reversed after removing the studies by Pan et al. (22) or Qin (20), indicating that the robustness of the results was poor. The inconsistent results of the two studies above were likely because different blood collection times and instrument models were used in the blood gas analysis. In summary, more studies are needed to identify the relationship between PaO2 and bronchiectasis in patients with COPD. The chance of contact with medical staff and patients in the same hospital increased after longer hospitalization, resulting in a greater risk of nosocomial infection. The hospitalized patients were more concentrated (29); therefore, the length of hospital stay directly affects the possibility of bronchiectasis in COPD. If patients with COPD were admitted to hospital within the past year, they might have had poorer disease control and acute exacerbation. The acute exacerbation of COPD resulted in repeated injuries to the lung tissue, leading to more severe airflow obstruction, which was susceptible to bronchiectasis (30). Thus, the disease should be strictly controlled according to the medical advice given to avoid admission for acute exacerbations to reduce the possibility of bronchiectasis. A longer duration of symptoms in COPD is a critical indicator of disease deterioration. Long-term clinical symptoms relieved the patient's resistance. The incidence of bronchiectasis was found to increase due to bronchial infection and the secretions blocking the airway (21). In conclusion, a longer duration of symptoms and hospital admissions within the past year were risk factors for bronchiectasis in COPD. The results of indicators such as purulent sputum, CRP, WBC, and N% were significant. Nonetheless, the results were reversed after removing some studies, indicating that the robustness of the results was weak. Some biases may exist in different clinical analysis instruments, and more rigorous studies are needed to identify these indexes.
In addition to the above indicators, the results including smoking index, BMI, mucous sputum, ESR, Hb, and FIB were not significant. However, the results were all reversed in the sensitivity analysis, and more clinical studies are required for supplementary verification. The smoking index may have something in common with smoking history, which tends to aggravate airway inflammation in COPD and increase the incidence of bronchiectasis. A study (31) has shown that low BMI is accompanied by a decrease in muscle mass, which may lead to depression in the strength of the respiratory muscles. Hu X et al. (32) proposed that COPD and bronchiectasis should have a high commonality in clinical symptoms, pathophysiology, and other aspects. The social burden and psychological pressure of patients were increased with the severe airway limitations related to bronchiectasis in COPD. Therefore, we should be familiar with the risk factors for bronchiectasis in patients with COPD. This will help ensure the early prevention, detection, and treatment of bronchiectasis in patients with COPD. Thus, to reduce their risk of bronchiectasis, patients with COPD should quit smoking and drinking alcohol, maintain a balanced diet, and prevent infection. We should devote equal attention to each complementary risk factor. The articles were strictly selected according to the inclusion and exclusion criteria. This study set the sources from which the authors received the diagnostic criteria for COPD and bronchiectasis. However, the final result might be affected by the interference of some factors, and there are several limitations in this meta-analysis, such as uncertainty bias in the secondary data, a limited number of articles, small total sample size, and unpredictable differences between sample sizes.
In conclusion, the presented results can be valuable to the medical community. The strengths of this review and meta-analysis include the inclusion of articles that assessed the quality of evidence evaluation using the GRADE approach. However, more studies with larger sample sizes are required. Furthermore, a multi-center case-control study is required to identify the risk factors scientifically and comprehensively for bronchiectasis in COPD. This study can be beneficial in guiding clinicians to formulate targeted prevention and treatment measures. This paper can provide recommendations for improving survival and quality of life and reducing the psychological, family, social, and medical burdens of patients with COPD and clinical guidance for reducing the incidence of bronchiectasis in patients with COPD.
AUTHOR CONTRIBUTIONSZhang XX was responsible for the topic selection and manuscript drafting. Zhang XX and Zhang HY contributed to the data acquisition and analysis. All authors contributed to the data interpretation and critical revisions of the manuscript. Pang LJ and Lv XD were responsible for the final decisions on data extraction and the quality assessment. Lv XD was responsible for funding and controlling the project.
This project was supported by the Natural Science Foundation of China (No:81373579, No:81403290), High-Level Innovation Team of Liaoning Province’s “plan of rejuvenating Liaoning talents” (XLYC1808011).
No potential conflict of interest was reported.