The goal of the present study was to estimate the risk ratio of herpes zoster among systemic lupus erythematosus patients after disease onset compared with a cohort of patients without systemic lupus erythematosus over a three-year period.
METHODS:A nationwide population-based cohort study using the National Health Insurance Research Database identified 10,337 new cases of systemic lupus erythematosus as the study cohort. In addition, 62,022 patients without systemic lupus erythematosus, who were matched for age, gender, and date of systemic lupus erythematosus diagnosis, were used as the comparison cohort. These cohorts were followed-up for three years. A Cox proportional hazard regression was performed to estimate the risk ratio of herpes zoster, with adjustments for age, gender, level of insurance, urbanization level, geographic region, comorbid medical conditions, average daily dosage of corticosteroids, and the use of immune-modulation agents.
RESULTS:Compared to patients without systemic lupus erythematosus, the crude risk ratio and adjusted risk ratio of herpes zoster among systemic lupus erythematosus patients were 7.37 (95% confidence interval 6.75-8.04) and 2.45 (95% confidence interval 1.77-3.40), respectively. Stratified by gender, the adjusted risk ratio of herpes zoster was 2.10 (95% confidence interval 1.45-2.99) in women and 7.51 (95% confidence interval 2.89-19.52) in men. Stratified by age, the adjusted risk ratio peaked in systemic lupus erythematosus patients who were aged 18 to 24 years (risk ratio 8.78, 95% confidence interval 3.08-24.97).
CONCLUSION:Based on nationwide population-based data, there is an increased risk of herpes zoster in systemic lupus erythematosus patients compared with non-systemic lupus erythematosus patients, particularly among males and patients aged 18 to 24 years. Further research on the associated risk factors for herpes zoster in systemic lupus erythematosus patients is needed.
Herpes zoster (HZ) is characterized by debilitating painful vesicular eruptions in a dermatomal distribution that are caused by the reactivation of a latent varicella zoster virus (VZV) infection.1 This disease is commonly observed in the elderly, in immuno-compromised hosts, and in patients with systemic lupus erythematosus (SLE).2–5 Cutaneous vesicles can be complicated by a bacterial super-infection, and the dissemination of HZ may be life-threatening.3 Post-herpetic neuralgia in the affected areas is the most common complication, which can persist for months or years. The incidence of HZ in the general population is 1.2 to 4.9 cases per 1000 person-years, depending on the ethnic group.6–7 The reported incidence of HZ in SLE patients is significantly greater than the incidence in the general population, ranging from 16 to 22 cases per 1000 patient-years in large cohort studies.2–3,8
The greater incidence of HZ infection in SLE has been shown to be related to abnormal T-cell-mediated cytotoxicity that is aggravated by the concomitant use of glucocorticoids and immuno-suppressants.4,9–10 Disease activity, lupus nephritis, and positive anti-Sm antibodies have been reported to be valuable risk factors for HZ in SLE patients.5 However, although the greater frequency of HZ infection in SLE patients is well recognized, a population-based cohort study has not yet been conducted. Moreover, the impact of age and gender on the risk of HZ in SLE patients remains unclear.
The purpose of the present study was to examine the estimated risk ratio for HZ among SLE patients in the three-year period after disease onset compared to that of a cohort of patients without SLE during the same time period using the Taiwanese National Health Insurance Research Database (NHIRD).
PATIENTS AND METHODSData sourceThe data source for the present study was the NHIRD, which includes inpatient and ambulatory care claims from 1996 to 2006. The National Health Insurance Program of the Bureau of National Health Insurance (BNHI) was implemented on March 1, 1995 and has since covered more than 98% of the population. The BNHI's computerized comprehensive database, including all of the medical claims for ambulatory care services and hospitalization, can facilitate a nationwide population-based cohort study. The NHIRD established a registry system for "Catastrophic Illnesses", including cancer, chronic mental illness, end-stage renal disease, congenital illness, and several autoimmune diseases such as SLE. The BNHI performs routine validations of the diagnoses by reviewing the original medical charts of all of the patients who applied for catastrophic illness registration. In Taiwan, the American College of Rheumatology classification criteria for SLE was used to validate the SLE diagnosis using the versions published in 1982 or 1997 based on the timing of making SLE diagnosis. Because the present study enrolled SLE patients solely from this particular registry, the accuracy of the SLE diagnosis was not a concern.
The NHIRD established a representative database from the entire set of enrollees by randomly selecting 1,000,000 subjects. The present study used all of the claims data for these 1,000,000 subjects during the period of 1996 to 2006 to select a comparison cohort of patients without SLE. This cohort matched the study cohort for age, gender, and the date of the first ambulatory visit after the SLE diagnosis.
Because the NHIRD consisted of de-identified secondary data that were released to the public for research purposes, the present study was exempted from a full review by the Internal Review Board.
Study samplesThe present study was a retrospective cohort study that consisted of a study cohort and a comparison cohort. The study cohort consisted of SLE patients (International Classification of Diseases, 9th Revision, Clinical Modification [ICD9-CM] Code 710.0) in Taiwan from the registry of catastrophic illness patients during the period of 1996 to 2006. The index date for the study cohort was identified as the date of the first-time ambulatory care visit that had a diagnosis code for SLE during that period. To identify new SLE cases, those who had an index date before January 1, 1998 were excluded. The SLE patients who were followed-up for less than three years were also excluded from the present study. To minimize the possibility of enrolling SLE patients with an episode of HZ before the index date or with a diagnosis code for HZ for prescribing medications for post-herpetic neuralgia after the index date, patients with any ambulatory visit for HZ (ICD9-CM code 053) within one year before the index date were excluded from the present study. The study cohort consisted of 10,337 SLE patients.
The comparison cohort was selected from a 1,000,000 representative cohort. Patients with an SLE diagnosis in any of the claims data during the time period of 1996 to 2006 were excluded from the present study. Patients from the registry of beneficiaries (six for every patient in the study cohort) were then randomly extracted as the comparison cohort, matching the study cohort for the age at SLE onset (i.e., 1-17, 18-24, 25-34, 35-44, 45-54, 55-64, >64 years), gender, and the year of the index date. The date of the first-time ambulatory visit within one year of the index date was selected as the index date for the comparison cohort. Patients with less than three years of ambulatory visit records after the index date were excluded from the present study. Patients with an ambulatory visit for HZ within one year before the index date were also excluded. The comparison cohort included 62,022 patients without SLE.
The potential confounding variables included the level of insurance as the economic index, the urbanization level, the geographic region (northern, central, southern, or eastern) of the patients, the types of medications, the presence of co-morbid medical diseases, and the Charlson co-morbidity index (CCI), as adapted by Deyo.11 The level of insurance was transformed to ordinal variables according to the 25th, 50th and 75th percentiles. In Taiwan, the urbanization levels were stratified into seven clusters, ranging from level one (most urbanized) to level seven (least urbanized).12 However, because the patient numbers in levels five through seven were small in both cohorts, these three levels were combined into one level (level five). The presence of prescription medication that was adjusted in the regression model included the average daily steroid dose (i.e., 0, <10, 10-20 and >20 mg prednisolone equivalent) and the use of azathioprine, cyclophosphamide, mycophenolate, cyclosporin, methotrexate, or hydroxychloroquine.
Diabetes mellitus (ICD9-CM code 250), lymphoma (ICD9-CM codes 200-202), leukemia (ICD9-CM codes 204-208), breast cancer (ICD9-CM codes 174-175), liver cancer (ICD9-CM code 155), and HIV/AIDS (ICD9-CM code 042) were co-morbid medical disorders because previous studies have suggested that these disorders are risk factors for HZ.7 The CCI was also calculated for the adjustments for possible confounding co-morbidities. Using the diagnosis codes in two or more ambulatory claims that were made six months before or after the index date, the co-morbidities were identified, and the CCI was calculated.
Statistical analysisTo examine the unadjusted comparisons, a t-test was used for the normally distributed continuous variables, and a Pearson χ2 test was used to analyze the categorical variables. The correlations between the variables were analyzed using Pearson's correlation coefficients. The Kaplan-Meier method was performed to estimate the three-year HZ-free survival rates, and the log-rank test was used to examine the differences in the risk of HZ between the two cohorts. Cox proportional hazard regressions were used to compute the crude and adjusted risk ratios (RR) with a 95% confidence interval (95% CI). A two-tailed p<0.05 was considered to be statistically significant. All of the analyses were conducted using the Statistical Package for the Social Sciences (SPSS) for Windows, Version 13.0 (SPSS, Inc., Chicago, Illinois).
RESULTSThe socio-demographic data between the study and comparison cohorts are shown in Table 1. The female to male ratio was 9:1, and the mean age (±SD) was 34.8±14.3 years for the study cohort and 34.8±15.0 years for the control cohort (p = 0.678). Approximately two-thirds of the patients were aged 18 to 44 years. The distributions of the insurance levels, urbanization levels, and geographic regions of residence were significantly different between these two cohorts.
Demographic data of patients with systemic lupus erythematosus (SLE) and of the control cohort.
| SLE patients(n = 10337) | Non-SLE Patients (n = 62022) | p-value | |
|---|---|---|---|
| Variable | |||
| Gender | 1.000 | ||
| Male | 1052 (10.2) | 6312 (10.2) | |
| Female | 9285 (89.8) | 55710 (89.8) | |
| Age groups, years | 1.000 | ||
| <18 | 955 (9.3) | 5730 (9.3) | |
| 18-24 | 1844 (17.8) | 11064 (17.8) | |
| 25-34 | 2724 (26.4) | 16344 (26.4) | |
| 35-44 | 2342 (22.7) | 14052 (22.7) | |
| 45-54 | 1438 (13.9) | 8628 (13.9) | |
| 55-64 | 650 (6.3) | 3900 (6.3) | |
| >64 | 384 (3.7) | 2304 (3.7) | |
| Urbanization level | <0.001 | ||
| 1 | 3824 (37.0) | 25220 (40.7) | <0.001 |
| 2 | 3444 (33.3) | 20612 (33.2) | 0.867 |
| 3 | 1497 (14.5) | 7929 (12.8) | <0.001 |
| 4 | 921 (8.9) | 4911 (7.9) | 0.001 |
| 5 | 651 (6.3) | 3350 (5.4) | <0.001 |
| Geographic region | <0.001 | ||
| Northern | 4943 (47.8) | 33484 (54.0) | <0.001 |
| Central | 2745 (26.6) | 13889 (22.4) | <0.001 |
| Southern | 2524 (24.4) | 13783 (22.2) | <0.001 |
| Eastern | 125 (1.2) | 866 (1.4) | 0.130 |
| Insurance level (NT$) | <0.001 | ||
| 0-6360 | 3047 (29.5) | 15307 (24.2) | <0.001 |
| 6361-15500 | 2319 (22.4) | 15782 (25.4) | <0.001 |
| 15501-20300 | 2583 (25.0) | 15437 (24.9) | 0.830 |
| >20300 | 2388 (23.1) | 15766 (25.4) | <0.001 |
Values are the number of patients (percentage), unless otherwise indicated.
The co-morbid medical conditions and prescription medications of the two cohorts are shown in Table 2. The study cohort exhibited greater proportions of patients with lymphoma, leukemia, liver cancer, and renal disease. In addition, greater proportions of patients were found to receive medication for systemic corticosteroids, azathioprine, cyclophosphamide, methotrexate, cyclosporin, and hydroxychloroquine compared to the control cohort. The study cohort had a significantly greater CCI; however, this cohort had a lower proportion of patients with diabetes mellitus.
Comparison of co-morbidities and prescription medications between the SLE patients and the non-SLE controls.
| SLE Patients(n = 10337) | Non-SLE Patients(n = 62022) | p-value | |
|---|---|---|---|
| Variables | |||
| Co-morbid medical conditions | |||
| Diabetes | 218 (2.1) | 1565 (2.5) | 0.012 |
| Lymphoma | 22 (0.2) | 18 (0.0) | <0.001 |
| Leukemia | 9 (0.1) | 10 (0.0) | <0.001 |
| Breast cancer | 17 (0.2) | 154 (0.3) | 0.104 |
| Liver cancer | 11 (0.1) | 18 (0.0) | <0.001 |
| HIV/AIDS | 0 (0.0) | 2 (0.0) | 0.564 |
| Charlson co-morbidity index, mean ± SD | 7.83 ± 1.22 | 0.23 ± 0.81 | <0.001 |
| Prescription medication | |||
| Systemic corticosteroid | 9695 (93.8) | 30254 (48.8) | <0.001 |
| Prednisolone equivalent dose (mg/day) | <0.001 | ||
| No use | 648 (6.3) | 32046 (51.7) | |
| 0-10 | 6588 (63.7) | 29946 (48.3) | |
| 10-20 | 2300 (22.3) | 26 (0.0) | |
| >20 | 801 (7.7) | 4 (0.0) | |
| Azathioprine | 3661 (35.4) | 29 (0.0) | <0.001 |
| Cyclophosphamide | 2287 (22.1) | 106 (0.2) | <0.001 |
| Methotrexate | 859 (8.3) | 140 (0.2) | <0.001 |
| Cyclosporin | 250 (2.4) | 28 (0.0) | <0.001 |
| Hydroxychloroquine | 8179 (79.1) | 199 (0.3) | <0.001 |
Values are the number of patients (percentage), unless otherwise indicated.
Among the SLE patients, age was found to be negatively correlated with average daily corticosteroid dose (Pearson correlation coefficient -0.118, p<0.001), and age was found to be positively correlated with CCI (Pearson correlation coefficient 0.106, p<0.001) (data not shown). Thus, the younger groups of SLE patients were administered larger average daily doses of corticosteroids (Table 3).
Average daily prednisolone (Pd) equivalent dose and Charlson co-morbidity index (CCI) for the SLE patients and non-SLE controls, as stratified by age.
| Pd equivalent (mg/day) | CCI | |||
|---|---|---|---|---|
| SLE Patients | Non-SLE Patients | SLE Patients | Non-SLE Patients | |
| Age, years | ||||
| <18 | 13.53 | 0.05 | 7.89 | 0.04 |
| 18-24 | 10.44 | 0.06 | 7.76 | 0.06 |
| 25-34 | 9.26 | 0.07 | 7.70 | 0.09 |
| 35-44 | 7.21 | 0.08 | 7.72 | 0.21 |
| 45-54 | 6.49 | 0.13 | 7.94 | 0.46 |
| 55-64 | 5.41 | 0.20 | 8.20 | 0.66 |
| >64 | 5.54 | 0.16 | 8.48 | 1.01 |
Values are the means of the groups.
The incidence rates with a 95% CI for HZ were 37.7 (35.5-40.0) cases per 1000 patient-years in the SLE patients and 5.1 (4.8-5.4) cases per 1000 patient-years in the non-SLE controls (Table 4). In the controls, the age-specific incidence rates were higher in the older groups, irrespective of gender. In contrast, among the SLE patients, the incidence rates peaked in the youngest group (<18 years), regardless of gender.
Incidence rates (number of cases per 1000 patient-years) of herpes zoster in the SLE patients and non-SLE controls, stratified by age and gender during the three-year follow-up period*.
| SLE Patients | Non-SLE Patients | |||||
|---|---|---|---|---|---|---|
| All | Female | Male | All | Female | Male | |
| Age, years | ||||||
| All | 37.7 | 37.6 | 39.2 | 5.1 | 5.2 | 4.7 |
| <18 | 58.1 | 57.8 | 59.8 | 2.2 | 2.4 | 1.3 |
| 18-24 | 37.5 | 38.8 | 27.6 | 3.2 | 3.3 | 2.5 |
| 25-34 | 30.0 | 29.5 | 35.4 | 2.9 | 2.8 | 3.8 |
| 35-44 | 37.7 | 35.4 | 39.2 | 4.8 | 4.9 | 3.9 |
| 45-54 | 42.5 | 43.7 | 31.0 | 10.1 | 10.5 | 7.0 |
| 55-64 | 34.1 | 32.1 | 49.2 | 10.0 | 10.0 | 9.7 |
| >64 | 46.9 | 45.1 | 53.8 | 12.8 | 13.0 | 12.0 |
Patients with SLE had a significantly greater risk of HZ than the patients in the control cohort (RR 7.37, 95% CI 6.75-8.04, p<0.001) (Table 5). After adjusting for the possible confounding factors that are shown in Tables 1 and 2, the SLE patients had a significantly greater risk of HZ than the control cohort (RR 2.45, 95% CI 1.77-3.40, p<0.001). After stratifying by age, the adjusted RRs for HZ in the SLE patients compared with those of the non-SLE controls reached statistical significance in most of the age groups and peaked in those aged 18 to 24 years. Stratified by gender, the male SLE patients exhibited larger RRs for HZ than the female SLE patients (RR 7.51, 95% CI 2.89-19.52 vs. RR 2.10, 95% CI 1.48-2.99).
Crude and multivariate adjusted risk ratios (RRs) with 95% confidence intervals for herpes zoster in the SLE patients and non-SLE controls, as stratified by age and gender, during the three-year follow-up period.
| Crude RR of HZ | p-value | Adjusted RR of HZ | p-value | |
|---|---|---|---|---|
| Age, years | ||||
| all | 7.37 (6.75-8.04) | <0.001 | 2.45 (1.77-3.40) | <0.001 |
| <18 | 25.81 (18.08-36.84) | <0.001 | 4.71 (1.41-15.70) | 0.012 |
| 18-24 | 11.81 (9.32-14.97) | <0.001 | 8.78 (3.08-24.97) | <0.001 |
| 25-34 | 10.43 (8.46-12.86) | <0.001 | 3.04 (1.26-7.33) | 0.013 |
| 35-44 | 7.40 (6.14-8.93) | <0.001 | 2.26 (1.06-4.82) | 0.034 |
| 45-54 | 4.19 (3.46-5.09) | <0.001 | 1.79 (0.88-3.65) | 0.108 |
| 55-64 | 3.45 (2.54-4.69) | <0.001 | 2.90 (1.12-7.49) | 0.028 |
| >64 | 3.65 (2.58-5.17) | <0.001 | 2.33 (0.79-6.87) | 0.126 |
| Gender | ||||
| Female | 7.27 (6.63-7.96) | <0.001 | 2.10 (1.48-2.99) | <0.001 |
| Male | 8.35 (6.33-11.02) | <0.001 | 7.51 (2.89-19.52) | <0.001 |
Multivariable regression models adjusted for the variables listed in Tables 1 and 2, including gender, age, insurance level, urbanization level, geographic region, co-morbidities, Charlson co-morbidity index, and prescription medications.
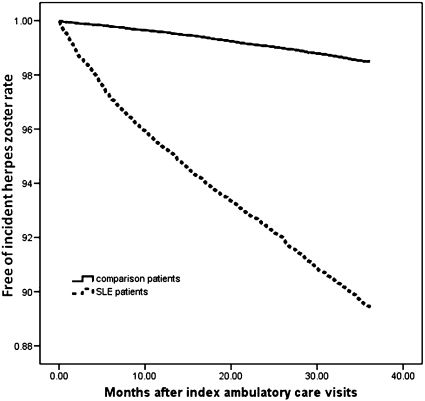
A log-rank test revealed that the SLE patients had a significantly lower risk of three-year HZ-free survival rates than the non-SLE controls (p<0.001). The duration between the index date and the date of HZ onset for the SLE patients ranged from one day to 1094 days, and the median and mean (± SD) duration were 436 days and 476±331 days, respectively. The survival curves for the two cohorts, which were determined using the Kaplan-Meier survival analysis, are illustrated in Figure 1.
DISCUSSIONThe present study has determined the incidence occurrences for HZ and the risk ratios for HZ among patients with SLE compared with those without SLE using nationwide population-based data. This study is the largest and first nationwide population-based cohort study regarding HZ infections in SLE patients. The incidence rate for HZ among the non-SLE patients (5.1 cases per 1000 patient-years) is consistent with those among the normal population that were reported in previous studies (1.2 to 4.9 cases per 1000 person-years).6–7 However, the incidence rate for HZ among the SLE patients in the present study (37.7 cases per 1000 patient-years) was larger than those among the SLE patients in previous cohort studies (16-22 cases per 1000 patient-years).2–3, Using an administrative database instead of reviewing medical records prevents underestimation. The adjusted RR for HZ in the SLE cohort was 2.45, which is in agreement with a previous study in Taiwan that reported that patients with HZ had a rate ratio of 2.12 for co-morbid SLE compared with that of controls.7 However, this result was further adjusted for the presence of prescription medication and the CCI, which can minimize the potential confounding effects of concomitant medications and other co-morbidities.
Previous studies have indicated that the incidence of HZ infection increases with age in the general population.7,13 The risk of HZ is greatest in subjects who are over 80 years of age and lowest in those who are younger than 20 years of age.13 In addition, the annual risk of HZ infection increases significantly in the geriatric population, with approximately two-thirds of individuals who are over 60 years developing HZ.14 Consistent with data from previous studies, the incidence rate among patients without SLE is greatest in subjects who are older than 64 years of age and lowest in subjects who are younger than 18 years of age. However, the current study reveals that the incident rate for HZ is greater in groups of younger SLE patients. Possible explanations for this finding include an increase in the SLE disease severity, larger doses of corticosteroids and/or immuno-suppressive agents, or a larger number of co-morbid conditions in younger SLE patients. Given the positive correlation between age and CCI in the current study, the last explanation is less likely to be the the cause of increased rate of HZ among younger SLE patients. The elderly lupus population has been reported to exhibit a greater number of benign disease courses,15–16 and physicians may prescribe lower doses of corticosteroid for elderly SLE patients because of a lower level of disease activity or a higher risk of drug-related complications, such as steroid-induced diabetes, atherosclerosis, or immuno-suppressive related infection. Thus, the first two reasons are more likely than the last one to lead to the larger incident rates that have been observed in younger SLE patients.
The crude RR for HZ is greater in young SLE patients and peaks in the youngest group of these patients. However, after an adjustment for the presence of prescription medication and co-morbid conditions, this trend seems to disappear. The adjusted RR for HZ is greatest in the SLE patients who are aged 18 to 24 years and lowest in those aged 45 to 54 years. It is not known whether this finding is related to SLE severity.
Previous studies have demonstrated that men have a lower incidence rate of HZ than women.12 However, the present study indicates that the incidence rate for HZ in male SLE patients is not lower than that in female SLE patients (39.2 cases per 1000 patient-years vs. 37.6 cases per 1000 patient-years). Compared with non-SLE patients, the adjusted RR for an HZ infection in male SLE patients (RR 7.51, 95% CI 2.89-19.52, p<0.001) is also greater than that in female SLE patients (RR 2.10, 95% CI 1.48-2.99, p<0.001). Despite these conflicting data, male SLE patients exhibit a greater level of renal involvement and a worse prognosis19–21 and may have an increased disease severity and a higher risk of HZ infection.
In Taiwan, the universal varicella vaccine was introduced for children who were born after mid-2002. However, all of the study subjects in the present study were born before August of 2002; therefore, the relative risk of HZ infection among SLE patients compared with non-SLE patients may not be influenced by the universal varicella vaccination policy in Taiwan. The finding of a higher risk of HZ in SLE patients raises the issue of whether active immunization should be used in these patients. A randomized controlled trial has shown that zoster vaccine, a live attenuated vaccine, is effective in preventing HZ and post-herpetic neuralgia in the elderly.17 However, there is limited evidence regarding the vaccine's use in SLE patients.18 Concomitant immuno-suppressive agents may reduce the efficacy of vaccines, and whether there are complications associated with this vaccination in the pathogenesis of SLE remain unclear.18 Further studies are needed to investigate the safety and efficacy of the varicella vaccination in SLE patients.
The present study has three advantages. First, the use of an administrative database prevents under-reporting of the number of medical visits.22 Previous studies on the incidence of HZ in SLE patients have mainly used medical record reviews at the selected institutions.2–5, Second, this nationwide population-based study avoids a selection bias. Third, the present study has adjusted for many important confounding factors, including age, gender, economic status, prescribed medication, and co-morbidities. Although race was not included in these adjustments, greater than 98% of Taiwan's residents are of Chinese Han ethnicity. This homogenous population makes the results of the present study unlikely to be confounded by race. However, this population also limits the generalization of the study results to other ethnic groups.
The present study also has several limitations. First, the accuracy of the diagnoses, which are based on the administrative data reported by physicians, may be a concern. Although the Bureau of National Health Insurance routinely samples patient charts to randomly cross-check the claims from all of the hospitals, a bias due to miscoding and misclassification may occur. Second, the lack of information about self-treatment with over-the-counter medications in the NHIRD may lead to underestimation of HZ. Third, an information bias may arise if the SLE patients have a greater tendency to visit physicians instead of using self-treatment with over-the-counter medications for cutaneous problems than the non-SLE patients. Another information bias may occur if physicians are more alert to the diagnosis of HZ in patients with SLE than in patients without SLE. Lastly, the administrative database cannot offer information about the SLE disease severity, which may also be a confounding factor for the results of the present study.
In conclusion, this nationwide population-based study shows that the relative risk of developing HZ in SLE patients after disease onset in a three-year follow-up period is 2.45 compared with that of non-SLE patients. Among the SLE patients, the RR for HZ is greater in males and peaks in those aged 18 to 24 years. Further investigation of the associated risk factors for HZ in SLE patients is needed.
We thank the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan, ROC for assistance with the statistical analyses. We thank the Bureau of National Health Insurance, Department of Health, which provided the National Health Insurance Research Database, and the National Health Research Institutes that managed the database.