To detect seroconversion of hepatitis B vaccine and antibody waning 3 years after vaccination in children immunized according to the World Health Organization schedule and its relationship to the mother’s serostatus during pregnancy.
METHODS:A serological study was carried out in São José dos Campos. Blood samples from pregnant women were taken for hepatitis B marker serology. To evaluate seroconversion in infants born to these women, serology was performed 1 month after they were vaccinated with recombinant vaccine. Another group of children was evaluated 3 years after being immunized.
RESULTS:Among 224 pregnant women, 0.9% were positive for hepatitis B surface antigen, 8.0% for antibodies to the surface antigen, and 4.5% for antibodies to the virus core. Seroconversion among 174 infants was as follows: absent in 18 children (10.35%), low level in 15 (8.62%), intermediate level in 26 (14.94%), and a high level (good response) in 115 (66.09%). Antibody positivity after 3 years was as follows: absent in 8 children (7.92%), low level in 51 (50.5%), intermediate level in 20 (19.8%), and high level in 22 (21.78%). Considering the age that the vaccine was administered, a significant proportion of non-seroconverters was found among children who had received the complete 3-dose schedule before 9 months (P = 0.023). Another factor that significantly contributed to the lack of seroconversion was the presence of any serological marker for hepatitis B during pregnancy (P = 0.044).
CONCLUSIONS:Data gathered in this work show that the immunization schedule for hepatitis B in low or moderate prevalence areas should be revised in order to optimize seroconversion.
Determinar a soroconversão vacinal da hepatite B em crianças no primeiro ano de vida e sua relação com a soropositividade das mães e avaliar a concentração de anticorpos 3 anos após a vacinação.
MÉTODOS:Estudo sorológico, realizado na cidade de São José dos Campos com amostras de sangue coletadas de gestantes para testar marcadores de Hepatite B. Para avaliar a soroconversão em crianças nascidas dessas mulheres, realizou-se sorologia mês após a última dose de vacina. Em outro grupo de crianças, imunizadas há 3 anos, foi feita a mesma sorologia.
RESULTADOS:Entre 224 gestantes, 0,9% foram positivas para o antígeno de superfície, 8,0% para anticorpos contra este antígeno e 4,5% para anticorpos contra o “core” viral. A soroconversão de 174 crianças, um mês após a última dose da vacina, foi ausente em 18 crianças (10,35%), baixa em 15 (8,62%), intermediária em 26 (14,94%), e houve boa resposta em 115 (66.09%). A soropositividade de 101 crianças vacinadas há 3 anos, foi ausente em 8 (7,92%), baixa em 51 (50,5%), intermediária em 20 (19,8%), e alta concentração de anticorpos em 22 (21,78%). Considerando a idade da vacinação, encontrou-se uma proporção significante de crianças soronegativas entre vacinadas antes dos 9 meses (p=0,023). Contribuiu significativamente para a baixa soroconversão a positividade de qualquer marcador sorológico durante a gravidez (p=0,044).
CONCLUSÃO:Os dados reunidos neste trabalho mostram que o calendário vacinal para a Hepatite B em regiões de baixa ou moderada prevalência poderia ser revisado para otimizar a soroconversão.
Hepatitis B is one of the world’s most serious infectious diseases. It is estimated that over 350 million people worldwide are chronic hepatitis B virus (HBV) carriers.1,2
Studies show that of all infected persons, 25% have acute hepatitis with jaundice, and 6% to 10% have chronic hepatitis.3 Of them, 40% die each year from HBV-related liver diseases in the USA.3,4
Between 35% and 40% of all HBV infections diagnosed worldwide every year result from vertically transmitted cases. The risk of infecting their children is increased among women found seropositive for both hepatitis B surface antigen (HBsAg) and precore antigen (HBeAg), an indicator of high HBV titers.5 In an attempt to reduce the spread of this virus, in 1991, the WHO recommended the introduction of HBV vaccination into the Programme of Immunization in all countries.6
The prevalence of hepatitis B is variable around the world.7 It is greater in high population density areas, such as south-east Asia and sub-Saharan Africa, and in isolated areas, such as Alaska, the Amazon, and some islands of the Pacific Ocean.8–10
In Brazil, the prevalence is moderate (2% to 7% in most part of the country), with the peak of infections occurring around 25 years of age.11 But in the Amazon, some authors have found a prevalence of 24.6% to 61.79%.9,10
According to the Brazilian Health Authority, hepatitis B vaccine was officially used for the first time in 1989 during a National Vaccination Day in an endemic area of eastern Amazon forest.
Since 1992, the Brazilian Health authority has implemented the vaccine program, giving priority to endemic areas, such as the States of Acre and the Amazon and for high-risk groups, such as persons with occupational risk throughout the country. The States of Santa Catarina, Espírito Santo, and Paraná implemented the immunization program against hepatitis B in 1993, and the Federal District implemented it in 1995.
The vaccine was recommended for all states of Brazil in 1996, targeting all children less than 1 year of age. But, in 1997 the amount of vaccine was insufficient to immunize according to the 1996 strategy. Only in 1998 did the stock of vaccines reach an adequate level to guarantee national coverage, and from then on immunization was regularly achieved, according to health authorities.
Several studies have been conducted in endemic areas for HBV. In these areas, the dynamics of anti-hepatitis B antibodies may be influenced not only by vaccination but also by natural exposure to HBV (natural booster).12 But, in an area in which the prevalence of HBV decreases, or with a lower prevalence, such as is found in Southern Brazil, the dynamics of antibodies against surface antigen (anti-HBs) and the persistence of the protection afforded by the hepatitis B vaccine may differ from what is observed in other studies.
The aim of this study was to detect the immediate seroconversion following HBV vaccination of children 1 month after the last dose for a community attended by the routine immunization programme of a Basic Health Unit in Brazil, to determine the seroprevalence of HBV markers and the prevalence of HBV carriers among pregnant women, and to determine the anti-HBs levels in the first cohort of children immunized by the official programme, in an attempt to contribute to better understanding of the effects of immunization in a population with moderate prevalence of hepatitis B infection.
METHODS2.1. Population and Study DesignThis study was carried out in São José dos Campos, a city of São Paulo State, Brazil. The city has an area of 1,102 km2 and is located just north of the Tropic of Capricorn (23o 13’ 53” latitude south, 45° 51’ 21” longitude west), 84 km from São Paulo City, the State capital. Its population is about 534,000 habitants, 95.1% in the urban area. The annual growth rate is around 1.89%. The city of São José dos Campos is strategically placed along the major road and rail links between the two largest Brazilian cities, namely, São Paulo and Rio de Janeiro, and is part of is known as the Latin America economic development pole.13
The economic activities in São José dos Campos include industrial facilities for aircraft, motorcar, pharmaceutical, telecommunications, health consumables, electro-electronics and photography. The average annual per capita income is BRL $15,000.00 (circa US $ 7,000.00). The lowest 8.75% of the population have an annual income of BRL $2,400.00 (circa US $ 1,000.00), and 18.26% have an income of BRL $ 4,800.00 (circa US $ 2,000.00) per year.14
This study was performed at the Basic Health Unit (BHU) of the borough of Campo dos Alemães; it is one of the 42 BHU in town, and serves approximately 21,800 people for assistance with clinical, pediatric and gynecologic primary care and prenatal care.
To check seroconversion and antibody concentration against the surface antigen (anti-HBs) a transverse serological study was performed with children immunized since 1998, the year in which the official programme was introduced. Two groups were established: the first cohort comprised children immunized after 6 months of age, and the second cohort, with children immunized according to the current vaccination schedule.
The recent seroconversion study was conducted in children born to women who attended in the prenatal program of the BHU of Campo dos Alemães.
From June 2000, pregnant women selected from a list of women with a positive b-HCG test were contacted and invited to participate in this study. A database was built with their complete names, record numbers, addresses, and predicted day of delivery. All participants were informed of the purpose of the study, and a written informed consent was subsequently obtained.
Blood samples from pregnant women were collected during the period from August 2000 to June 2001, approximately 6 weeks before delivery, for determination of HBV markers.
The newborn babies were examined monthly by the pediatrician conducting this study (TMR) as part of first-year pediatric routine. From each child’s record, the weight, height, and adverse events related to vaccination were recorded in our immunization database. These infants were vaccinated according to the schedule proposed by the Brazilian Health Authority. One month after the last dose of hepatitis B vaccine, blood samples were collected from the children and tested for anti-HBs.
Parents of the children assisted at Campos dos Alemães who were immunized against hepatitis B from October 1998 until August 2001 were contacted to enter this study, constituting a group in which we analyzed the antibody concentration more than 1 year after vaccination.
2.2. VaccineThe vaccines used routinely in our country are bought, handled, and administered by central and local health authorities. During this study, the children received any of these yeast-derived hepatitis B vaccines: Euvax B recombinant®, LG Chemical Ltda. Pharmaceutical Div., Seoul, Korea—241 doses; Engerix-B®, Smithkline Beecham, Belgium—495 doses; and Hepavax-Gene®, Korea Green, South Korea—89 doses.
Each dose of this vaccine was given in the musculus vastus lateralis of right thigh, in a dose of 0.5 mL (10 m.
The vaccine was given during the first month of life and then 1 month and 6 months after the first dose.
2.3. Blood collection and serologyBlood samples were collected by venipuncture with butterfly and syringe designed for children and through a vacuum collecting system designed for pregnant women.
Samples were left at room temperature for 2 hours and then centrifuged at 3000 rpm for 15 minutes. Serum was separated from the clot, then frozen to and preserved at - 20 °C until being transported to the university laboratory.
Sera were transported to the city of São Paulo inside a thermal box with recycled ice (Gelo-X®, Adiquima Ind Com Adit Ltda., SP, Brazil) to keep the temperature between 2 and 8 °C, and then stocked in a freezer at -20 °C, at the Laboratory of Immunosuppressive Investigation (LIM 01), Department of Pathology, Hospital das Clínicas, São Paulo University Medical School until laboratory analysis.
Serum was tested by enzyme immunoassay technique, using commercial kits as follows: HBV surface antigen (HBsAg) by Murex HBsAg Version 3®(Abbott, UK); antibodies against surface antigen (anti-HBs) by ETI-AB-AUK-3® (DiaSorin, Italy) and Murex anti-HBs® (Abbott, UK); and antibodies against core antigen (anti-HBc), using Hepanostika® anti-HBc Uni-Form (Organon Teknika, Holland).
The variables collected for the study were: (i) gender, (ii) weight and height at birth and at the last dose of vaccine for calculation of body mass index (weight/height2), (iii) the Capurro value, (the most used method for determination of the gestational age, in weeks, from clinical and neurological data), (iv) age of child at first and third dose of vaccine, (v) vaccine brand(s), (vi) interval between last dose and blood collection, and (vii) determination of the HBV markers of the mother (anti-HBs, anti-HBc, and HBsAg).
2.4. Data management and analysisData were collected and organized using a spreadsheet software (MS-Excel®, MS-Office 97®, Microsoft, USA). Statistical tests were performed using EPI-INFO version 6.0 STATCALC and EPITABLE modules, CDC, USA & WHO, Switzerland, and MINITAB version 13.1, Minitab Inc., USA.
Categorical variables were analyzed by the χ2 test corrected by the Mantel-Haenszel technique where applicable, adopting 5% as the level of significance.
Anti-HBs concentration was analyzed by 1-way ANOVA to test differences among groups classified by the time from the last vaccine dose.
RESULTSFrom June 2000 to June 2001, 507 pregnant women were contacted and invited to participate in this study. Blood samples were taken from 224 pregnant women.
Among the 224 pregnant women, the following distribution of ages was observed: 24 (10.71%) were 14 through 17 years of age; 60 (26.78%) were 18 through 21 years; 51 (22.77%) were 22 through 25 years; 45 (20.09%) were 26 through 29 years; 23 (10.27%) were 30 through 33 years; 12 (5.36%) were 34 through 37 years; and 9 (4.02%) were 38 through 41 years of age.
Regarding the questionnaire answered by those 224 pregnant women, the following information was found: previous history of diagnosed hepatitis B infection (0.4%), hepatitis B infection in family members (3.1%), history of alcoholism (0.4%), history of previous blood transfusions (4.0%), and history of use of non-intravenous illicit drugs (4.9%). None of the patients admitted a history of previous use of intravenous illicit drugs. Two patients (0.9%) were vaccinated against hepatitis B because they had jobs that require this immunization.
Seroprevalence among pregnant women was found to be 8.0% for anti-HBs (18 in 224), 4.5% for anti-HBc (10 in 224), and 0.9% for HBsAg carriers (2 in 224).
The 224 participants delivered 226 newborns. Of them, 6 (2.7%) died, 3 (1.3%) had the permission to enter the study suspended by their parents, 13 (5.8%) moved from the city, and 3 (1.3%) gave wrong addresses.
In the final account, 174 children were sampled and serology was performed. All of them received the complete course of hepatitis B vaccination.
Details of the time of administration of the first vaccine dose were available for these 174 children as follows: 74 (42.5%) received the vaccine within 48 hours after birth and 100 (57.5%) had their first dose after this time. This occurred because the vaccine is administrated soon after birth in only 1 hospital. The other children received their first immunization at the Health Basic Unit.
Among the 174 infants, 85 (48.8%) were female and 89 (51.2%) were male. The distribution of race was as follows: 13 (8.44%) black; 96 (62.34%) white; and 45 (29.22%) mulatto.
Vaccinees were observed for fever, injection site reactions, and systemic complaints. No side effects of the vaccine were observed, with only minor limited local reactions at the site of administration.
Seroconversion was as follows: absent in 18 infants (10.35%), low level (10 to 100 mIU/mL) in 15 (8.62%), intermediate level (101 to 500 mIU/mL) in 26 infants (14.94%), and high level (good response) (> 501 mIU/mL) in 115 vaccinees (66.09%). The average concentration was 740.87 mUI/mL (± 524.79 mUI/mL).
Cross tabulation of children’s serostatus for anti-HBs and their mother’s positivity for any HBV serological marker presented a significant association (P = 0.044), showing that seroconversion was lower in children from positive mothers (Table 1).
No significant difference was found between the antibody response to anti-HBV vaccine and weight and height (body mass index) at the time of the third dose.
To check the persistence of anti-HBs concentration in the group of children immunized after 1998 by the national programme, 101 children 1 through 4 years old were sampled among 1520 children who had received the HBV vaccine in Campo dos Alemães BHU. Among those 101 children, 49 (48.5%) were girls and 52 (51.5%) were boys.
Of them, 32 children were from the first group vaccinated in 1998 (first cohort), sampled after an average of 32 months from the last vaccine dose. Data on anti-HBs antibody concentration was obtained in 69 children sampled 11 to 30 months after the last dose of HBV vaccine (second cohort).
The antibody response to hepatitis B vaccine of the first cohort was as follows: no one was negative for anti-HBs, 14 (43.8%) had low levels of antibodies (10 to 100 mIU/mL), 9 (28.1%) had intermediate levels (101 to 500 mIU/mL), and 9 (28.1%) had high levels (good responses) (> 501 mIU/mL). Antibody concentration varied between 35.19 to 1973.87 -mUI/mL. The mean antibody concentration was 406.38 mUI/mL (±504.50 mUI/mL).
The serological responses of the second cohort was as follows: antibodies were absent in 8 (11.6%), 37 (53.6%) had low levels (10 to 100 mIU/mL), 11 (15.9%) had intermediate levels (101 to 500 mIU/mL), and 13 (18.8%) had high levels (good responses) (> 501 mIU/mL). Antibody concentration varied between 1.56 to 1257.41 mUI/mL. The mean antibody concentration was 218.50 mUI/mL (±304.94 mUI/mL).
Table 2 shows the results of the 3 groups studied for seropositivity for anti-HBs.
To analyze antibody waning from the last dose of vaccination and the seroconversion related to age, data from the 3 groups were added together to form a single group.
If we consider the age, in months, at which the children received the first dose of the hepatitis B vaccine, we observe that the likelihood of seroconversion increases if it is given after the second month of life (Table 3).
Moreover, if we categorize the children by the age at which they received the third dose of HBV vaccine, we observe that the seroconversion also increases if this last dose is given after the 6.5 months of age (Table 4). We elected to stratify into 3 levels, namely: up to 6.5 months, 6.5 though 9 months, and after 9 months. This strategy stems from the age at which the first dose of hepatitis B vaccine was given: if the child received the first dose soon after birth, he/she received the last dose around 6 months of age; but if the first dose was administered after the second month of life, the last dose was given after 9 months of age. It should be noted that the interval between the doses is specified, and these doses tend to be administered in the interval proposed or later, never in a shorter period of time.
When all the children were stratified into 2 groups by the age at which they received the third HBV vaccine dose, using 7 months of age as the cut-off point, a significant difference in seroconversion in favor of the older children (P = 0.022) was observed. In this case, children immunized by the official proposed calendar did not benefit as much as those for whom immunization was delayed to a later age (Table 5).
Age at the third HBV vaccine dose versus seroconversion, by stratification into 2 age groups
| Mother’s serostatus for HBV markers | Children negative for anti-HBs | Children positive for anti-HBs | Total |
|---|---|---|---|
| negative | 14(8.86%) | 144(91.14%) | 158 |
| positive | 4(25%) | 12(75%) | 16 |
| Total | 18(10.35%) | 156(89.65%) | 174 |
χ2 = 4.0 (P = 0.044) corrected by Mantel-Haenzel.
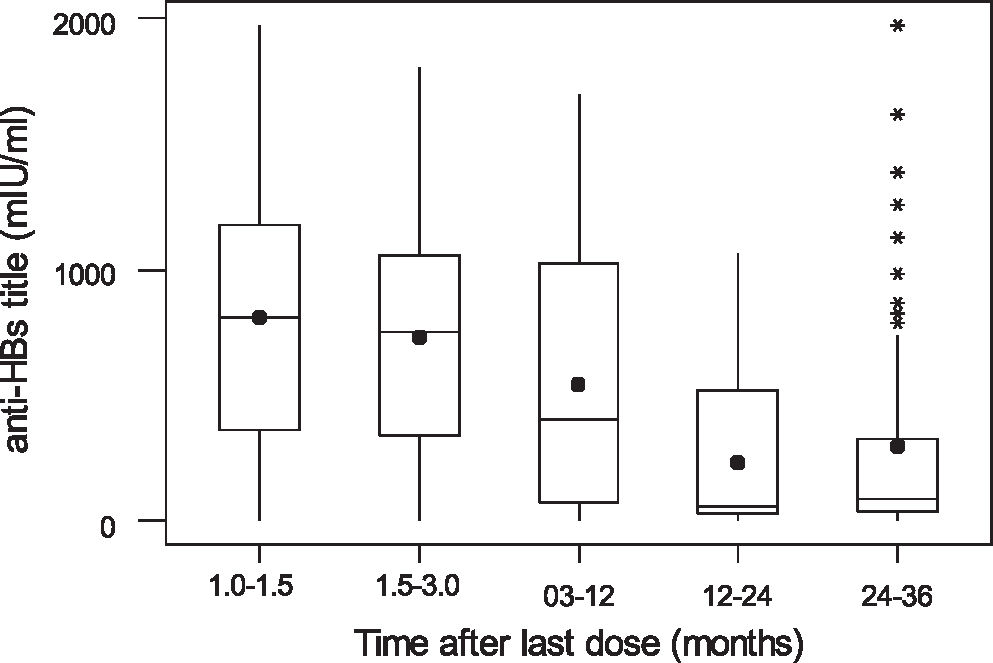
We classified time elapsed after last dose of anti-HBV vaccine was given into 5 categories, namely: 1 through 1.5, 1.6 through 3, 3.1 through 12, 12.1 through 24, and 24.1 through 36 months. The analysis of variance showed that there is a significant difference among these groups in respect to anti-HBs concentration (P < 0.001) (Figure 1).
DISCUSSIONThe main objective of HBV vaccination is to prevent the chronic carrier state and its associated morbidity and mortality, especially in areas where the prevalence is higher, posing risks to the infants.15 The vast majority of reports have been about the experience with HBV vaccine in these types of areas.
There are few studies that adequately address the effect of HBV vaccination in areas where the prevalence is moderate to low, and therefore the circulation of the virus is correspondingly low, which may cause the outcomes of the immunization in the long run to be potentially different from outcomes in countries or areas with a high HBV prevalence.
The optimal age for immunizing a population must take into account some characteristics of the target population, such as the best seroconversion time, the age-dependent force of infection, as well as antibody waning after vaccination and its potential effect on weakening immunity, and consequently the necessity of re-vaccination schemes.
The HBV prevalence in Southern Brazil is low to moderate. As shown by our data, 1% of pregnant women are infected and carrying HBV, being a risk for vertical or perinatal transmission. The prevention this type of transmission seems to have been improved by serological screening carried out since the year 2001 during the prenatal official programme. Women positive for HBV and their newborns are treated with hepatitis B immunoglobulin and vaccination as soon as the baby is delivered.
Considering this epidemiological scenario, it is pertinent to question the practice of vaccinating all infants soon after birth without investigating seroconversion at this age, or the efficacy of the resulting immunity as protection against hepatitis B infection.
Seroconversion studies have shown a good response among newborns and infants. In a recent study done in São Paulo State, Brazil, full-term newborns had a high rate of seroconversion following vaccination with recombinant hepatitis B.16 In that study, 98% (95% CI = 91.6-99-9) seroconverted, with 1.8% having low (<10 mIU/mL), 26.3% having intermediate (10-100 mIU/mL), and 71.9% having good (>100 mIU/mL) anti-HBs titers, with a mean anti-HBs titer of 537.5 mIU/mL. Our experience with the immune response in full-term babies is different, in that we found a greater percentage with low antibody levels and a higher percentage of good responses. In our study, we found that 1 month after the last HBV vaccine was given, seroconversion was low (<10 mIU/mL) in 10.35%, intermediate (10-100 mIU/mL) in 8.62%, and high (good) (> 100 mIU/ml) in 81.03% of the neonates, with a mean concentration of 740.87 mIU/mL (± 524.79 mIU/mL).
The previous study cited above was conducted in an University Hospital, where subjects and materials are under better control when compared with native community with its populational heterogeneities in terms of immunological response and application of vaccination procedures.
This could possibly explain differences in the proportion of low responders to Hepatitis B vaccine.
It must be noted that vaccine products come from different vaccine laboratory manufacturers and countries. Although this fact may have introduced heterogeneity in immunological response among vaccinees, our results are still valid and relevant for evaluating the actual herd immunity obtained from the immunization programme. In other words, health authorities must be aware that varying vaccine products to be applied in immunization will influence the population protection against a given disease. Consequently, it is important to keep a surveillance program for evaluating the real outcome of a vaccination strategy.
It is important to emphasize that seroconversion obtained in this routine immunization service was around 90% among children who had received the HBV vaccine before 9 months of age. This phenomenon could result in an accumulation in the future of 10% per year of individuals susceptible to HBV infection, exposing this population to a greater risk, because they believe they are immunized.
Our data indicates that seroconversion is maximized after 9 months of age.
Moreover, considering that there is a significant decay in the antibody concentration, it is possible that individuals immunized early in life will be susceptible and at increased risk when adolescents or during adulthood, when the incidence of hepatitis B is greater.
In conclusion, it seems to be inadequate to maintain the immunization programme against hepatitis B soon after birth as it is presently scheduled. It is our opinion that HBV vaccine should be given after 9 months of age in low and moderate HBV-endemic areas.
ACKNOWLEDGEMENTSThe authors wish to express their sincere gratitude to the families that participated in the study, as well as to Ronielley William Pereira, Dulcinéia Margarida Landim, Maria Alice de Sales, Márcia Gimenez Aguiar da Silva, and all those from the staff of the BHU Campo dos Alemães for their help during the study in dealing with children and mothers. We are also in debt to Dr Élide Zélia Santo from Pasteur Laboratory of São José dos Campos for lab facilities during blood sample processing. TMR and RSA were partially supported by CNPq.