The present study was designed to determine the normal range of serum beta 2-microglobulin (Sb2M) levels among healthy volunteers in Brazil. Levels of Sb2M are elevated in human immunodeficiency virus (HIV)-infected patients and have been shown to be the best predictor of HIV infection status and of some malignant disorders, especially multiple myeloma. In order to achieve its optimal use in Brazilian clinical diagnosis, an adequate reference interval study was performed for Sb2M IMMULITE® assay, based on the fact that its reference range limits were evaluated among European populations.
METHODSNinety-six healthy blood donors were evaluated, and Sb2M levels were measured by chemiluminescent enzyme immunoassay using the IMMULITE® automated analyzer.
RESULTSA normal range of Sb2M values, established by a nonparametric statistical method, was 1.05 to 3.9 mg/mL, with the upper limit being higher than that reported elsewhere.
CONCLUSIONSThis study presented new data indicating that there is a significant difference between the current reference limits for Sb2M IMMULITE® assay and those found in Brazil, providing evidence that significant differences in range of normal values may occur among different populations, and that these new values should be considered for Brazilian individuals.
O objetivo do presente estudo foi determinar valores de referência de β2-Microglobulina sérica (Sb2M) em voluntários saudáveis. Sabe-se que tal parâmetro apresenta-se elevado em pacientes infectados com o vírus da imunodeficiência humana (HIV) e tem se mostrado melhor marcador da infecção por HIV e de desordens malignas, especialmente mieloma múltiplo. De forma a se obter o melhor diagnóstico clínico, um intervalo de referência adequado de Sb2M para a população brasileira foi determinado empregando-se o ensaio IMMULITE®; já que este tem como parâmetro uma faixa de referência determinada a partir de populações européias.
MÉTODOSNoventa e seis doadores de sangue saudáveis foram avaliados e os valores de Sb2M foram medidos por método enzima imunoensaio quimiluminescente usando analisador automatizado IMMULITE®.
RESULTADOSOs valores de Sb2M, estabelecidos por método estatístico não paramétrico, apresentaram-se entre 1.05 e 3.9 mg/ml, sendo que o limite superior obtido foi maior que o relatado em outros trabalhos.
CONCLUSÃOEste estudo apresentou novos valores, indicando que existe uma diferença significante entre os limites de referência de Sb2M disponibilizados para o IMMULITE® e os encontrados no presente trabalho, evidenciando a ocorrência de variações importantes entre diferentes populações e que novos valores devem ser considerados para brasileiros.
Beta 2-microglobulin (b2M) a protein found in all nucleated cells, is associated with histocompatibility class 1 antigens on cell surface membranes (particularly abundant on lymphocytes and monocytes).1 It was first discovered in the urine of patients with renal failure.2 Because of its low molecular weight (11,800 daltons), 95% of all free b2M in plasma is eliminated by glomerular filtration, and 99.9% of it taken up by proximal tubular cells. In the presence of a normal renal filtration rate, elevated serum b2M (Sb2M) levels indicate high b2M production or release3. Upon activation of the immune system, both B- and T-lymphocytes actively release b2M into circulation from where it is later eliminated via glomerular filtration and tubular reabsorption.4 Increased Sb2M concentrations have been found in patients with blood cells dyscrasias, including multiple myeloma,5,6 malignant lymphoproliferative disorders,7 myeloproliferative disorders,8 certain viral infections such as HIV infection9, cytomegalovirus, non-A and non-B hepatitis, and infectious mononucleosis.10 The level of Sb2M is also used as an important prognostic factor in patients with multiple myeloma5,6 and HIV infections.9
The normal reference range for Sb2M in healthy individuals found in the literature was determined using North American and European populations (DPC Immulite® assay). In an attempt to determine the normal range of Sb2M levels among asymptomatic seronegative African individuals, Piwowar et al. (1995)10 found that 50% of these subjects had abnormal values (mean values 2.35 mg/mL). In another study, the mean Sb2M value among healthy volunteers was 1.36 mg/mL.11 In this report, we describe Sb2M values in healthy Brazilian subjects from a group of blood donors at the Hematology and Hemotherapy Center of Santa Catarina (HEMOSC).
SUBJECTSBlood donors, men and nonpregnant women ranging from 25 to 50 years of age, were included in the study. All individuals underwent a clinical assessment evaluated by HEMOSC, including questionnaires and screening laboratory tests according to Brazilian legal criteria. All volunteers fulfilling these criteria gave verbal and written consent to have an additional 5 mL of blood drawn for the study purpose. The experimental protocol was approved by the Ethical Committee of the University Hospital of Florianópolis, Federal University of Santa Catarina.
METHODSWhole blood was collected with a Vacutainer system in 10-mL dry tubes, and serum was separated by centrifugation. In order to eliminate lipid components, serum samples were ultracentrifuged at 18000 g for 20 min. All blood samples were obtained between 2:00 p.m. and 6:00 p.m. and were processed within 5 h after venipuncture. Samples were prediluted 1-in-41 in b2M Sample Diluent (IMMULITE® Beta–2 Microglobulin kit, DPC, USA) according to the manufacturer's instructions, and 5 mL were required for the assay procedure. Sb2M was measured by a solid-phase 2-site chemiluminescent enzyme immunoassay using the IMMULITE® Automated Analyzer (DPC, Los Angeles, USA).
Statistical analysisIn the reference range study for the DPC IMMULITE® assay, the mean value was 1.26 mg/mL. The National Committee for Clinical Laboratory Standards (NCCLS) addresses the use of a priori and a posteriori exclusion criteria to obtain a healthy samples.12 Therefore, all subjects with Sb2M values greater than 2.0 mg/mL were clinically reevaluated for value acceptance to ensure that every individual was “healthy”. Data were entered and analyzed with Microsoft Excel software. The mean and standard deviation values were calculated. The 95th-percentile reference ranges were determined by using 2.5 and 97.5 percentiles. According to the procedure recommended by the NCCLS, the observations were ranked according to size, and the 2.5 and 97.5 percentiles were obtained as the 0.025 (n + 1) and 0.975 (n + 1) ordered observations respectively (n= sample size). If the estimated rank values were not integers, linear interpolation was carried out.12
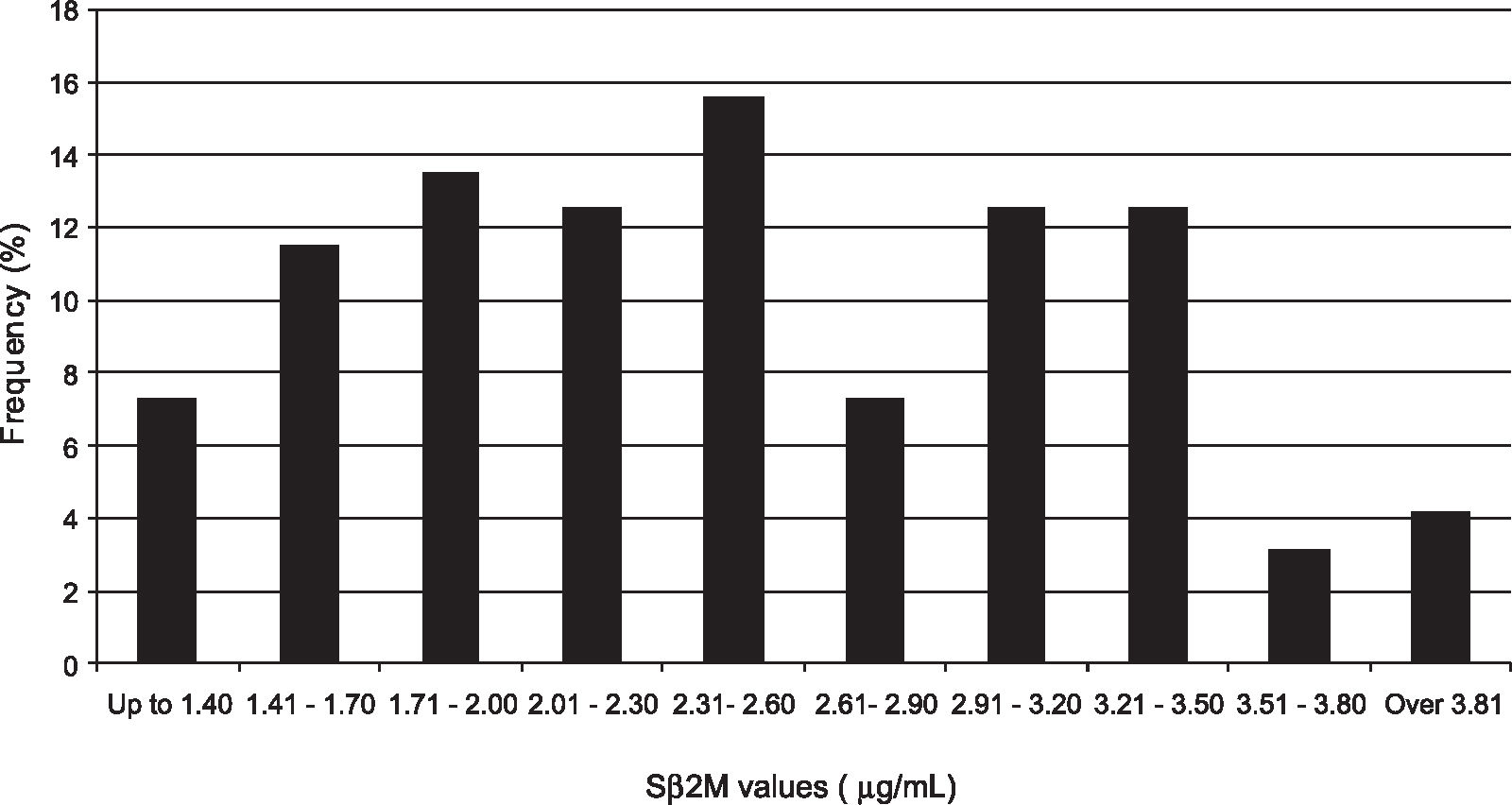
RESULTSNinety-six Brazilian blood donors, 60 men and 36 women, with mean age (yr) of 33, were included in the study. Values of Sb2M ranged between 1.05 and 3.9 mg/mL, as shown in Figure 1, and the mean was 2.46 mg/mL (standard deviation [SD] = 0.73). Therefore, using 97.5th percentile according to a routine nonparametric method,12 3.9 mg/mL was considered the upper normal limit. The range established was 1.3–3.9 mg/mL.
DISCUSSIONResearchers worldwide have reported the usefulness of Sb2M as a reliable maker in evaluating progression of HIV-infection and multiple myeloma.5–8,10,13 In HIV-infected subjects, Sb2M appears to be a better predictor of HIV infection status than CD4 counts and also a better predictor of survival, with increased Sb2M levels being associated with disease progression.10
Increased production and consequent release of Sb2M into body fluids have been reported in patients with multiple myeloma. The importance of these data for clinicians is based on the fact that myeloma plasma cells produce Sb2M. Therefore, it is assumed that Sb2M values could indicate not only the size but also the proliferative activity of the myeloma cells13 and could be accepted as a nonspecific maker of tumor mass.5,6,13,14 In addition, the prognostic role of Sb2M in other proliferative disorders has been confirmed by many authors,7,14,15 facilitating clinical assessment.15 On the other hand, it has been reported that in patients with low glomerular filtration rate, Sb2M levels were raised and laboratory findings should be interpreted with caution.6,7,13
Reference intervals are used clinically, together with additional information, as guidelines concerning the state of the patient. The reference range study for IMMULITE® Sb2M (1.0–1.7 mg/mL, mean 1.26 mg/mL) defined by the manufacturer was determined using serum samples from almost 800 adult volunteers, including both men and nonpregnant women, ranging from 20 to 70 years of age. The subjects were selected based on questionnaires. Samples were collected in France, Germany, the Netherlands, and Portugal, and values obtained were analyzed nonparametrically. The suggested upper limit was 1.7 mg/mL. These values may not be representative for Brazilian individuals. In the absence of adequate reference range limits for Sb2M derived from any Brazilian population, many investigators have been using data obtained from North American and European subjects that presented upper limits 2.3 times lower than that obtained in the present study.
The NCCLS recommends that nonparametric reference intervals be used and that the sample size, n, should consist of at least 120 values in order to compute a 95% reference interval, 2.5% and 97.5% points of the distribution12. This number is the minimum number of values needed for construction of the confidence intervals giving information about the accuracy of the calculated reference limits.
In this study, 96 samples were evaluated. Our goal was not to produce new reference intervals, but to bring more focus on the need for every laboratory to produce its own reference intervals for all parameters in spite of many difficulties, including practical ones and high expenses.
The present study indicates that significantly different ranges of Sb2M values may occur among different populations, suggesting that each Brazilian laboratory should establish its own reference limits.
We thank the Hematology and Hemotherapy Center of Santa Catarina and the Clinical Analysis Laboratory, University Hospital of Florianópolis for the samples and technical assistance. We are grateful to DPC IMMULITE® for providing the reagent set free of charge for this study and to Dr. Joanita Del Moral from the Hematology Division, University Hospital of Florianópolis.