Although schizophrenia affects both human genders, there are gender-dependent differences with respect to age of onset, clinical characteristics, course and prognosis of the disease.
METHODSTo investigate sex-dependent differences in motor coordination and activity as well as in cognitive and social behavior, we repeatedly tested female (n = 14) and male (n = 12) Fisher rats (postnatal days, PD 56–174) that had received intracerebroventricular injections of kainic acid as well as female (n = 15) and male (n = 16) control animals. The hippocampus was examined histologically.
RESULTSCompared to male controls, in the alcove test both female controls and female animals with prenatal intervention spent less time in a dark box before entering an unknown illuminated area. Again, animals that received prenatal injection (particularly females) made more perseveration errors in the T-maze alternation task compared to controls. Female rats exhibited a higher degree of activity than males, suggesting these effects to be sex-dependent. Finally, animals that received prenatal intervention maintained longer lasting social contacts. Histological analyses showed pyramidal cells in the hippocampal area CA3 (in both hemispheres) of control animals to be longer than those found in treated animals. Sex-dependent differences were found in the left hippocampi of control animals and animals after prenatal intervention.
CONCLUSIONThese results demonstrate important differences between males and females in terms of weight gain, response to fear, working memory and social behavior. We also found sex-dependent differences in the lengths of hippocampal neurons. Further studies on larger sample sets with more detailed analyses of morphological changes are required to confirm our data.
According to neurodevelopmental models, schizophrenia is caused by a prenatal dysfunction of neuronal migration and orientation occurring throughout the brain, especially in the hippocampus. Malformations of hippocampal neurons have been interpreted as a failure of orderly migration of neurons generated in the neuroepithelium that lines the walls of fetal cerebral ventricles.1 Although this process occurs during the second trimester of human pregnancy2,3 (which corresponds to day 18 in the rat), the symptoms of the disease, which are probably due to neurodevelopmental damage, do not appear till after adolescence. At this stage of development substantial changes in cognition, emotion, and behavior take place. In humans, the risk of schizophrenia is highest after puberty, from 20 to 35 years of age. In rats, adolescence occurs during postnatal day (PD) 43 to 65.
Schizophrenia is a complex disorder including symptoms such as hallucinations, delusions or an impoverished faculty of speech. These characteristics naturally cannot be comprehended in animals. Accordingly, animal models cannot be expected to mirror all aspects of the disease Nevertheless, they might be useful for studying the etiology and pathophysiology of schizophrenia. For example, it is possible to apply certain standardized test parameters in animals for examining motor activity, response to fear, working memory, and learning and social behavior. These effects can be compared to analogous symptoms in humans. Prenatal exposure to stress is known to alter many characteristics of adult rat behavior and, furthermore, marked sex differences are apparent4. Thus, prenatal exposure to stress has been used to develop animal models of schizophrenia.5,6 Prenatal infections by influenza viruses, Borna Disease Virus or Herpes simplex I virus, which are assumed to be involved in the etiology of schizophrenia, result in distinct neurobehavioral alterations in rats.7 Maternal obstetric complications are regarded as an environmental contribution to the risk of developing schizophrenia,8. Anoxia at birth, again, differentially affects the functional development and maturation of dopamine-related behavior in postpubertal rats.9,10 Current lesion animal models of schizophrenia 11,12 involving to inflict damage on the hippocampus of neonatal pups via kainic acid are based on the hypothesis of the complete maturation of the hippocampus proceeding 1 to 7 days after birth. Kainic acid, a so-called lesion tool, damages nerve cells without degrading nerve fibers projecting from other brain regions. The herefrom deduced results indicate that an early developmental hippocampal insult in the rat impairs behavioral, biochemical and cellular processes, mimicing many human schizophrenia symptoms.
Previous research revealed significant gender-dependent differences in schizophrenic patients. Negative symptoms and cognitive deficits more frequently affect male humans with severe structural brain and neurophysiological abnormalities.13–16 These differences are thought to mainly result from the effects of sex hormones along with neurodevelopmental and psychosocial sex differences.17–,19 This coincides with the observation that the manifestation of schizophrenia in women is delayed until menopause and its severity to be reduced by oestrogen20,21. Animal tests have also provided strong evidence for the influence of gonadal hormones. Andrade et al.22 showed that the hippocampal formation might be sensitive to the structure-organizing effects of gonadal steroids. This is in line with the results of Diaz-Veliz et al.23, who suggested a modulatory influence of estrogen on behavior that is possibly mediated by the activity of dopaminergic neurons. Indeed, thus prenatally treated male rats exhibited alterations resembling human schizophrenia symptoms as less weight gain, shorter cells in the hippocampus, and deficits in learning and memory.24 These results suggest that there are basic differences between male and female rats with respect to behavior and neural organization.
We assume that schizophrenia is a neurodevelopmental disease and that some of its characteristics are sex-dependent. The aim of the present study, which introduces a novel animal model for the etiology of schizophrenia, consisted in investigating the effects of prenatal invasive intervention (intracerebroventricular [icv] injection of kainic acid) on weight as well as motor, cognitive, and social behavior of female Fisher rats, and in comparing these to those previously obtained from male Fisher rats.24. Furthermore, histopathological examination of the hippocampal CA3 region (one of the most important control centers of the hippocampal circuit) was performed. These tests covered the critical period between late adolescence and early adulthood (from weeks 8 to 12). To investigate the possibility of habituation effects on behavior, the tests were carried out over a period of 6 months.
MATERIALS AND METHODSAnimalsSurgical procedures, details of behavioral tests, and histological preparation have been described previously24. Briefly, on day 18 of pregnancy, 13 female Fisher rats (Tierversuchsanlage Düsseldorf, Heinrich-Heine-University of Düsseldorf) were anesthetized with Hypnorm (0.1 ml/100 g body weight, i.m.) and Dormicum (0.1 ml, i.m.). After opening the abdominal wall, the uterus was lifted carefully from the abdominal cavity. The third brain ventricles (i.c.v.) of a total of 94 fetuses in utero received bilateral injections with 0.2 μl kainic acid (1 mg/2 ml NaCl 0.9%) via a cannula (0.33 × 13 mm/29Gx11/2). A Zeiss op-microscope was used for visual control of the procedure. To reduce leakage of amniotic fluid, the amniotic sac of each fetus was penetrated only once. Afterwards, the abdominal wall was closed and the dams were observed for 1 day before taking them back to their home cages.
We tested females which received a prenatal injection of kainic acid (n = 14) and non-treated female controls (n = 15) in comparison to equally treated males (n = 16) and male controls (n = 12). The offspring of 5 females without intervention (12 males and 15 females) served as control group. Males and females emanated from the same litters and were tested at the same time and under equal test conditions The respective results on the male rats were published by Sprick et al.,24, the proportion of males and females are displayed in Table 1.
Proportion of male (m) and female (f) newborn rats and pups surviving 1 week after birth for nontreated controls and prenatally-interventioned Fisher rats
| controls | prenatally interventioned rats | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pregnancy | 1 | 2 | 3 | 4 | 5 | ∑ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ∑ |
| fetuses | 9 | 6 | 3 | 8 | 8 | 12 | 7 | 3 | 9 | 6 | 71 | ||||||
| newborns | 5 | 5 | 6 | 4 | 7 | 27 | 5 | 3 | 0 | 7 | 0 | 11 | 7 | 1 | 9 | 6 | 49 |
| pups | 5 | 5 | 6 | 4 | 7 | 27 | 2 | 3 | 0 | 2 | 0 | 10 | 0 | 1 | 8 | 4 | 30 |
| m:f | 4:1 | 1:4 | 2:4 | 2:2 | 3:4 | 12:15 | 1:1 | 2:1 | 0:2 | 6:4 | 0:1 | 5:3 | 2:2 | 16:14 | |||
On PD 21, the pups were removed from their mothers and individually housed with free access to food and water. After postnatal day 42–48 (week 6), the animals were handled and weighed once per week until PD 168–174 (week 24). The behavioral tests were carried out every four weeks from PD 56–62 (week 8) until PD 168–174 (week 24), and conducted between 8:00 and 20:00 during the active night cycle of the animals. To avoid a possible habituation to the tests, different tests were applied during weeks 6 and 7.
Behavioral testsNeurological testsIn the rat, the grasping reflex and the contact reflex of the vibrissae are unconditional neuronal-mediated native reflexes, and their dysfunction can indicate a neuronal disturbance.
The grasping reflex of the two forelimbs was examined by: a) touching the palms with a bar, and b) holding the rat on its tail and slowly moving it downwards towards a table top. Orientation activity was analyzed via stimulating the right and left vibrissae with a Q-tip. Three scores were assigned for fully triggered reflexes, two for those only partially triggered, and one score for none.
Motor coordinationMotor coordination deficits are possible indicators for prenatal or infantile brain damage. Thus, the motor coordination of the rats was assessed via testing their ability to balance on a 1.0 m long and 1.0 cm wide bridge. Three scores were assigned for crossing the bridge without hesitation, two scores for stalling or only slowly crossing it, and one score for falling off the bridge.
Alcove testThe alcove test is a refined open-field exploratory test25,26 and serves as an indicator of anxiety and fear in rats. The rats initially were individually placed in a small dark box connected to a brightly lit open field and equipped with a guillotine door. After pulling up this door, the time span until leaving the box was measured. Each trial ended after 2 min and was repeated after 30 min; the scores of the two tests were summed.
T-mazeThe T-maze27 was employed as learning and working memory test. After starting food deprivation the previous day, the rats were supposed to enter the short arm of the maze containing food pellets. If the rats found the pellets on three consecutive trials, the food was placed in the second short arm. Altogether, 20 such runs were carried out. The test was denoted “correct” when the rat directly entered the arm containing the food, or “incorrect” when the animal entered the arm without. A “perseveration error” was noted when the rat entered the no-food arm on three consecutive trials. The total number of correct runs was counted.
Horizontal locomotor and home cage activityLocomotor activity28 is an important element of the spatial and target-oriented exploration behavior of the rat. This was tested in an open field with automatic measurement by photocells for periods of 30 min. Home cage activity, a locomotor test in which the animals remain in their own cage to decrease stress, was monitored by organizing the cage of each animal into a frame with photocells. The number of beam interruptions was measured during each 30 min test trial.
Social interactionRats are social animals requiring a high number of different social contacts. Social exploration includes sniffing the entire body of the partner which serves as olfactory recognition. In our study, social behavior was assessed by the duration of nose contact29 between the test animal and a partner rat in a time period of 1 min. As the test partners were either a male or female with strange adult control or a male or female sibling, four tests were carried out for each animal.
HistologyAt PD 175–182 (week 25), the rats were sacrificed and perfused with Karnovsky’s solution. The brains were removed, deeply frozen at −40°C and cut into 25-μm-thick serial slices. Every sixth slice was stained with cresyl violet, and the length of the pyramidal cells in area CA3 of the dorsal hippocampi was measured using a computerized system (Vids IV, AMS, United Kingdom). The length of each nerve cell was determined as the distance between the starting point of the axon and the dendrites. At least 500 cells (approximately 65 cells per slice) from each hemisphere of each brain were measured.
Statistical analysisAll data from the behavioral tests and the animals’ weights are presented as the means ± SEM. Three-factor ANOVA with GROUP and SEX as factors and WEEKS as the repeated measure factor were carried out to evaluate the differences between male and female rats. Unless otherwise specified, the data from the female rats were analyzed using two-way ANOVA with GROUP as the between-group factors and WEEKS as repeated measure factor. T-tests for independent samples with α-adjustment were carried out posthoc for single data points.
RESULTSThe results and statistical analyses of the tests with the male Fisher rats have been previously published.24
Survival rateOf 13 untreated pregnant Fisher rats, three died within 24 h after surgery due to infection or trauma. Their values could not be included in the evaluation of the survival rate of pups. Therefore, of 71 fetuses from 10 dams injected intracerebroventricularly with kainic acid in utero, 49 pups were born alive (69% survival rate). Of these pups, 30 (16 males and 14 females) survived the first week and were tested. Thus, we obtained a 42.3% survival rate after 7 days. The offspring of 5 untreated females (12 males and 15 females) served as control groups. The male-to-female proportion of the surviving pups is depicted in Table 1.
WeightsComparing the two sexes, we found differences for WEEKS [F(6,264) = 2774.12, p < 0.000001], GROUP*WEEKS [F(6,264) = 6.67, p = 0.000001], SEX*WEEKS [F(6,264) = 428.26, p < 0.000001] and GROUP*SEX*WEEKS [F(6,264) = 5.60, p = 0.000017]. As expected, male animals were heavier than the females; thus, we also found differences for GROUP [F(1,44) = 18.11, p = 0.00012], SEX [F(1,44) = 520.24, p < 0.000001] and GROUP*SEX [F(1,44) = 8.45, p = 0.006].
Female rats showed a continuous weight gain from week 6 to week 24 [F(6,132) = 1039,1, p < 0.00001]. We did not discover differences for WEEKS*GROUP [F(6,132) = 1.25, p = 0.29] or between the control and prenatal intervention group in females [F(1,22) = 1.44, p = 0.24].
In contrast to the weight of the male control and prenatally-interventioned groups, which differed significantly from week 6 to week 24, female animals showed no differences in weight gain over the tested period.
Neurological testsAll animals performed the two tests of grasping reflexes of the forelimbs. Male and female controls fully accomplished (category 1) the orientation reflex in weeks 8, 12, 16 and 20. However, only 80% of the females gave a correct response on the right side and 90% on the left side at week 24. Depending on the week of testing, between 73.3% to 87.5% of the male and 78.6% to 92.9% of the female prenatal intervention groups accomplished the test parameters. Accordingly, the reflex was either not fully accomplished or not achieved at all (category 2) by 12.5% to 26.7% of the males and 7.1% to 21.4% of the females with prenatal intervention. Distributions of intervention and control animals were similar over the two categories on weeks 8, 12, 16, 20 and 24 with p > 0.05 (Chi-square test, note that 50% or 66.7% of the expected frequencies were less than 5).
Motor coordinationIn contrast to 75% to 88.9% of the male controls, all female controls crossed the bridge without any problems (category 1). The remaining animals either displayed difficulties at crossing the bridge or fell off (category 2). Between 60% to 81.3% of the male and 84.62% to 100% of the female prenatal intervention groups successfully accomplished the test. Differences between the four groups over the tested period in weeks 8, 12, 16 and 24 (p = 0.19, p = 0.42, p = 0.22, p = 0.041 and p = 0.36, respectively) were not significant (Chi-square test, note that 50% and 66.7% of the expected frequencies were less than 5).
Alcove testThree-way ANOVA indicated no intersubject effects over time [F(4,152) = 1.32, p = 0.27], and no changes occurred as a function of sex [F(4,152) = 0.75, p = 0.56] or group [F(4,152) = 1.32, p = 0.27]; there was also no interaction for GROUP*SEX*WEEKS [F(4,152) = 0.73, p = 0.57]. In contrast, we found differences for GROUP [F(1,38) = 6.29, p = 0.017] and SEX [F(1,38) = 13.43, p = 0.001], but not for GROUP*SEX [F(1,38) = 2.62, p = 0.114].
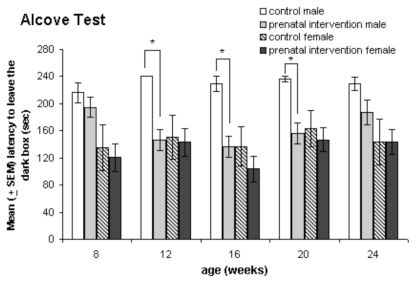
The female prenatal intervention group left the box quicker than the controls. However, statistical analyses showed no differences for WEEKS [F(4,72) = 1.28, p = 0.286], WEEKS*GROUP [F(4,72) = 6.46, p = 0.909] or GROUP [F(1,18) = 0.266, p = 0.612], in contrast to males, in which there were significant differences between the groups from weeks 12 to 20 (Fig.2).
Alcove test. The time (in seconds) spent in a dark box before exploring a brightly lit open field was measured at weeks 8, 12, 16, 20 and 24 for the male (n = 6) and female (n = 6) controls and male (n = 16) and female (n = 14) prenatal intervention groups. The two latencies, A1 and A2, were summed. The male prenatal intervention group and the females exited the dark box more quickly than the male controls over the test period of 24 weeks. The values of males differed at weeks 12, 16 and 20; male and female controls displayed differences at weeks 16, 20 and 24; *p ≤ 0.01.
Due to missing values from control animals on different testing days, an ANOVA for repeated measurements could not be carried out. Therefore, the results from female rats were evaluated using t-tests with p < 0.01. Female controls demonstrated superior performance vs. the prenatal intervention group (p = 0.006) via a higher number of correct responses only in week 12. By contrast, male controls and prenatally interventioned rats did not differ from each other throughout the complete test period.
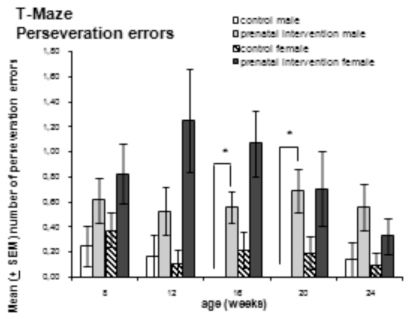
The number of perseveration errors is presented in Fig.3. Although prenatally interventioned females showed a high number of these errors (especially in weeks 12 and 16), these numbers did not significantly differ from those of female controls (p = 0.019 and p = 0.012, respectively). Contrastingly, male controls differed significantly from treated animals at weeks 16 and 20.
T-Maze perseveration errors. The number of perseveration errors of male (n = 13–16) and female (n = 12–14) animals that received prenatal intervention, and that of male (n = 5–8) and female (n = 9–13) controls was measured at weeks 8, 12, 16, 20 and 24. Male and female animals that received prenatal intervention demonstrated a higher number of perseveration errors than the controls. These differences were significant for females at weeks 12 and 16, and for males at weeks 16 and 20; *p ≤ 0.01
As expected, female animals exhibited a slightly higher locomotor activity compared to males. Nevertheless, three-way ANOVA only showed a change over the weeks for the intersubject effects (WEEKS [F(4,180) = 11.57, p < 0.000001], and there were no changes as a function of group (GROUP*WEEKS [F(4,180) = 1.26, p = 0.29] or sex (SEX*WEEKS [F(4,180) = 0.95, p = 0.44], or for the interaction GROUP*SEX*WEEKS [F(4,180) = 0.95, p = 0.44]. Although there was no difference for GROUP [F(1,45) = 1.73, p = 0.2] or GROUP*SEX [F(1,45) = 3.0, p = 0.091], there was one for SEX [F(1,45) = 45.5, p < 0.000001].
After 30 min of testing, female animals had different activity scores over the weeks [F(4,88) = 4.28, p = 0.003], with the highest activity scores in weeks 12 and 16, but no differences were apparent for WEEKS*GROUP [F(4,88) = 1.30, p =0.28] or GROUP [F(1,22) = 0.12, p = 0.73]. In general, female controls exhibited higher activity than their prenatally interventioned counterparts. This difference was not observed in the male animals. In summary, the results adumbrated the observed differences in locomotor activity to be due to sex-related variances over the test period.
Home cage activityIn general, female rats showed higher activity counts for home cage behavior than males. Three-way ANOVA did not reveal a change over the weeks (WEEKS [F(4,180) = 1.04, p = 0.39], or changes occurring as a function of group (GROUP*WEEKS [F(4,180) = 1.22, p = 0.31], sex (WEEKS*SEX [F(4,180) = 1.80, p = 0.13], or the interaction GROUP*SEX*WEEKS [F(4,180) = 1.33, p = 0.26]. While there was no difference for GROUP [F(1,45) = 0.00, p = 0.99] or GROUP*SEX [F(1,45) = 0.45, p = 0.51], there was a difference for SEX [F(1,45) = 10.7, p = 0.002].
Female controls exhibited slightly lower levels of activity in the home cage test than prenatal intervention animals. In contrast to males, which differed significantly from each other at week 12, statistical analyses did not detect any differences after 30 min of testing for females for GROUP [F(1,22) = 0.12, p = 0.73], for WEEKS [F(4,88) = 1.05, p = 0.39] or for GROUP*WEEKS [F(4,88) = 0.11, p = 0.98].
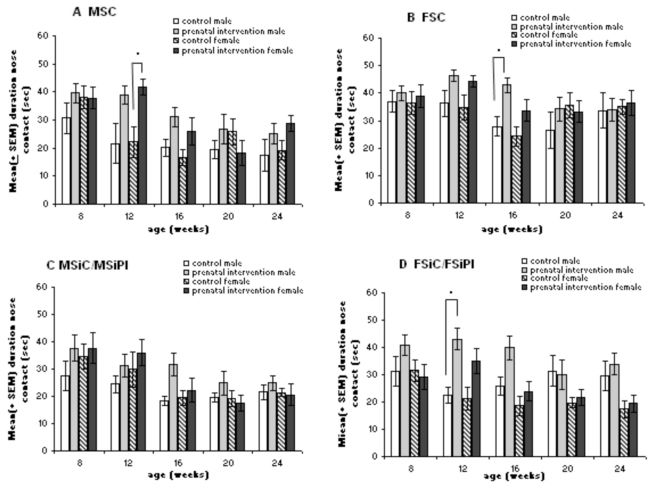
Social behaviorDue to missing values from control animals on different days of testing, ANOVA for repeated measures could not be carried out; however, it was possible to compare the groups using t-tests for independent samples. In general, female rats with prenatal intervention held their noses in contact with their respective partners (male and female strange rats, Fig.4 A and B; and male and female siblings, Fig.4 C and D) for longer periods than the female controls. This difference could also observed in the male groups, especially at weeks 12 and 16. However, statistical analysis revealed the differences between the female control and prenatally-interventioned groups to be only significant (p = 0.004) at week 12 and provided that the partner was a male strange animal. Males differed from each other at weeks 12 and 16 if the respective partner was a female. All other comparisons yielded p-values greater than 0.01.
Social interaction test. Nose contact within 60 seconds for male (n = 5–9) and female (n = 8–13) control and male (n = 12–13) and female (n = 9–11) prenatal intervention groups with different partners** was measured at weeks 8, 12, 16, 20 and 24. In general, male and female prenatal intervention groups showed a greater extent of social contact than controls, depending on the degree of kinship. At week 12, there were differences between male and female controls and male and female animals which received prenatal intervention with MSC and FsiC/FSiPI as partners, and at week 16 for the male rats with FsiC/FsiPI as partners; *p ≤ 0.01. **MSC = male strange control; FSC = female strange control; MSiC or MSiPI = male sibling control or prenatal intervention; FSiC or FSiPI = female sibling control or prenatal intervention.
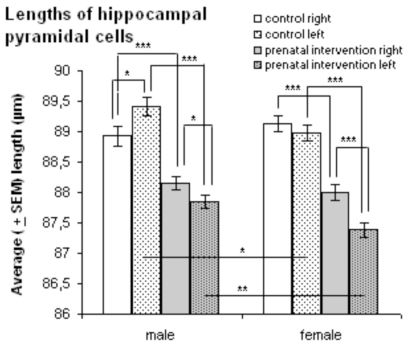
In total, 650 pyramidal cells from the CA3 region of the right and left dorsal hippocampi of each rat brain were stained with Cresyl violet, and their lengths were measured (Fig.7). The mean (± SEM) lengths of pyramidal cells from right hippocampi were 88.15 ± 0.11 and 88.01 ± 0.13 for male and female prenatally interventioned animals, respectively. The mean (± SEM) lengths for pyramidal cells from left hippocampi were 87.84 ± 0.11 and 87.39 ± 0.12 for male and female prenatal intervention groups, respectively. While the mean (± SEM) lengths for pyramidal cells from right hippocampi were 88.93 ± 0.16 and 89.13 ± 0.14 for male and female control groups, respectively, the values for cells from the left hippocampi were 89.41 ± 0.15 and 88.98 ± 0.13 for male and female controls, respectively.
T-tests for independent samples showed significantly longer cell lengths in both right (p = 0.001 for males and p < 0.00001 for females) and left hippocampi (p < 0.00001 for males and females) of controls compared to the prenatal intervention groups. Comparisons within groups showed both male and female prenatal intervention groups to have significantly longer pyramidal cells in right compared to left hippocampi (p = 0.05 and p = 0.004, respectively). Whereas male controls had significantly longer pyramidal cells in left hippocampi (p = 0.03), the mean length of cells in female controls was greater in the right hemisphere (although this difference was not significant; p = 0.42).
Cell lengths in right and left hippocampal areas of the male prenatal intervention group were greater than those in the female group; this difference was significant for the left (p = 0.0006), but not for the right side (p = 0.41). For male controls, only the lengths of the cells of the left hippocampus were significantly greater than the corresponding values of the females (p = 0.03). In the right hemisphere, cells of male controls were shorter than those of females, although this difference was not significant (p = 0.35).
DISCUSSIONTo avoid the influence of gonadal hormones, most previous studies have been restricted to male rats.6 However, schizophrenia affects both human sexes, and there exist known sex-dependent disparities in age of onset, clinical characteristics, and the course and prognosis of the disease. In our test trials, we discovered the behavior of female rats to differ from that of males with respect to body weight gain, learning and memory, social behavior and hippocampal neuronal changes. Similar differences have been observed in schizophrenic patients (see Table 2).
Effects of a prenatal intervention with kainic acid at day 18 of pregnancy on the offspring. We tested 12 male (m) and 14 female (f) controls, and 16 male and 15 female Fisher rats with prenatal intervention, from postnatal week 8 to week 24
| Male rats control:intervention | Female rats control:intervention | m:f | |
|---|---|---|---|
| Body weight gain | Significantly higher in controls | No difference | Higher body weight in males |
| Memory, learning, social information | Deficits in long-term memory of social information and working memory of treated rats | Deficits in long-term memory of social information and working memory of treated rats | More “correct response” and less perseveration errors in malesIncreased nose contact in males |
| Response to fear | Decreased fear in prenatally interventioned rats from week 12 to 20 | Minor decreased change in prenatally interventioned rats | Higher latencies of male controls |
| Hippocampal cell length | Decreased length in prenatally interventioned rats | Decreased length in prenatally interventioned rats | Differences between males and females in the left hemisphere |
Schizophrenia is related to disturbances in executive functions, memory, attention and motor functioning.30 Our analysis of the behavioral tests in rats indicated some differences to be due to sex-dependent animal-specific variations rather than being caused by prenatal intervention. Specifically, these differences were observed for horizontal locomotor activity, home cage behavior, neurological testing, and the motor coordination task. In line with our results, Silva-Gomez et al.31 reported higher activity levels for females than for males. Kanyt et al.32 observed untreated female rats to be more active than untreated males. Moreover, the activity levels of ovariectomized female rats were lower than those of the untreated females, and this deficit was reversed by hormonal priming. This suggests that the action of gonadal hormones may account for at least some of the different reactions of males and females in behavioral activity and coordination testing.
One interesting finding pertained to the difference in weight gain of rats between weeks 6 and 24. While male rats with a prenatal intervention showed lesser weight gain than their control counterparts, no such difference was observed in the respective female groups. Our results accord with those of Bhatnagar et al.33, who observed that the weight of male but not of female rats was significantly influenced by prenatal stress. Although little is known about the weight of schizophrenic humans before the onset of the disease, various studies34,35 have shown that adolescents with very low birthweight have an increased risk of developing psychiatric disorders by the age of 14 and that, before the onset of the illness, future male schizophrenic patients (16–17 years old) weighed less than the rest of the cohort. Wetterling et al.36 observed that the body weight of first-episode schizophrenia patients without neuroleptic treatment was lower than the one of the general population. Thus, further investigations are obliged to focus on differences in weight, particularly birth weight and weight gain during adolescence. Apparently, a lower weight entails a higher risk of developing the disease during the period between after puberty and early adulthood. Indeed, a lower-than-normal weight gain could perhaps be used as a parameter for diagnosis.
Patients with schizophrenia have pronounced deficits in working memory37,38 and learning39. We substantiated memory deficits not only in the T-maze alternation test (a test of learning and working memory), but also in social behavior. Female rats with prenatal intervention showed lower levels of correct responses, and the lowest scores were obtained at week 12. Unlike male and female controls, the prenatal intervention groups had higher levels of perseveration errors, and there was a habituation effect over time in females that was not present in males. The social behavior of rats remains unchanged during lifetime, thus we did not detect any age-related alterations.40 Nose contact is part of their social exploration behavior and serves as a means of olfactory identification.29 The increase in investigation behavior might be an indicator for animals with a prenatal intervention to have difficulties in recognizing their partners. This may reflect a deficit in the long-term memory of social information,28 and also the social withdrawal behavior as known from schizophrenic humans. Contrastingly, studies with neonatally lesioned rats31 showed sex-specific reductions in the duration of active social interactions. Thus, further investigations with refined parameters conducted over a longer test period are needed to clarify social behavior in our animal model.
Anxiety is a frequent but often unrecognized feature of schizophrenia associated with a severe level of disability41. In the alcove test, which tests an animal’s ability to enter and explore a novel, vast, brightly lit and open area in which access to a familiar compartment is permitted,26 as expected, male controls showed a tendency to remain in the box for the entire duration of the 2 min test period. The behavior of the male controls differs from the characteristics of prenatally interventioned males and females and appeared from puberty to early adulthood and remained apparent as late as week 20. However, there were no differences between female control and prenatal intervention groups. The residence time was as brief as the latency of the male prenatal intervention group. Rats usually avoid exploring a novel and brightly lit open field. We assumed prenatally interventioned males to exhibit less anxiety in the alcove test than male controls, whereas females with prenatal intervention showed a less distinct divergence from the female controls.
In addition to behavioral tests, we measured the length of the pyramidal cells in the hippocampal CA3 area. After 6 months of life, rats exhibited sex-specific differences in the cell morphology of both brain hemispheres. Moreover, there were differences between the control and the prenatally interventioned groups. Pyramidal cell atrophy has been proved in the post-mortem brains of schizophrenic patients42. It has been hypothesized that abnormalities in the hippocampal regions contribute to diminished GABAergic modulation in schizophrenia43, and that this may play a role in the cognitive dysfunction of the disease.44 In addition to prenatal intervention, social isolation of the single-housed rats45,46 and postnatal handling might also be stress factors possibly altering the morphology of hippocampal cells.47 Postweaning social isolation decreases long-term potentiation in the rat hippocampal pathways.48 Prolonged social isolation can increase the appetitive motivation for social play.49 The incidence of social interactions has been shown to be higher in individually housed male rats than in group-housed animals.50 According to several reports, postnatal handling can influence sex-dependent stress reactivity51 and this way attenuate anatomical and cognitive dysfunctions in rats.52 Neonatal novelty exposure affects sex differences in emotional reactivity53 and modulates hippocampal-dependent learning and cerebral lateralization54 as well as prenatal stress. Moreover, behavioral modification of the dams – as a reaction to treatment – causes sex-specific responses in the hypothalamic-pituitary-adrenal-axis of the offspring.55,56,57 Further investigations are needed to clarify the effects of these factors.
In summary, our animal model of prenatal intervention resulted in sex-dependent alterations in body weight, behavior, and hippocampal cell morphology of the offspring comparable to findings in schizophrenic patients. Our results confirm the hypothesis that effects of prenatal intervention occur during late adolescence and early adulthood, which is comparable to the time of onset of schizophrenia in humans.
We thank Dr. A Treiber, Director of the TVA, Heinrich-Heine-Universität, Düsseldorf and her assistants for their patient and expert assistance during the animal experiments. Dr. M. Jänner, Rheinische Kliniken, Düsseldorf, kindly shared her expertise in statistics with us.