We aimed to evaluate angiotensin receptor blocker add-on therapy in patients with low cardiac output during decompensated heart failure.
METHODS:We selected patients with decompensated heart failure, low cardiac output, dobutamine dependence, and an ejection fraction <0.45 who were receiving an angiotensin-converting enzyme inhibitor. The patients were randomized to losartan or placebo and underwent invasive hemodynamic and B-type natriuretic peptide measurements at baseline and on the seventh day after intervention. ClinicalTrials.gov: NCT01857999.
RESULTS:We studied 10 patients in the losartan group and 11 patients in the placebo group. The patient characteristics were as follows: age 52.7 years, ejection fraction 21.3%, dobutamine infusion 8.5 mcg/kg.min, indexed systemic vascular resistance 1918.0 dynes.sec/cm5.m2, cardiac index 2.8 L/min.m2, and B-type natriuretic peptide 1,403 pg/mL. After 7 days of intervention, there was a 37.4% reduction in the B-type natriuretic peptide levels in the losartan group compared with an 11.9% increase in the placebo group (mean difference, -49.1%; 95% confidence interval: -88.1 to -9.8%, p = 0.018). No significant difference was observed in the hemodynamic measurements.
CONCLUSION:Short-term add-on therapy with losartan reduced B-type natriuretic peptide levels in patients hospitalized for decompensated severe heart failure and low cardiac output with inotrope dependence.
The management approach for stable chronic heart failure has been established by guidelines (1) that were developed using data from randomized clinical trials. However, during heart failure, decompensation-specific interventions have not been well studied. Moreover, heart failure patients require hospitalization, and sometimes they are in critical condition, presenting problems such as a low cardiac output. In this context, fewer clinical trials have been performed.
The intravenous administration of inotropes may be necessary for heart failure patients with low cardiac output if intravenous vasodilator therapy is not possible (2). In end-stage heart failure, patients frequently become dependent on inotrope, which predicts greater mortality (3). Generally, these patients die if they cannot receive a heart transplant, either because of clinical conditions or because of the lack of available donor organs. Therefore, it is important to find alternative options so that inotropes can be withdrawn as soon as possible.
During decompensated heart failure, activation of the renin-angiotensin-aldosterone system persists, with increases in plasma renin activity and serum levels of angiotensin II and aldosterone, despite the use of angiotensin-converting enzyme (ACE) inhibitors (4). In advanced heart failure patients, a state of low cardiac output could be present during a decompensated period; consequently, the use of ACE inhibitors might not be adequate for controlling vasoconstriction (5).
Although many clinical trials have been performed to examine drug therapies for stable chronic heart failure, clinical trials are lacking for drug therapy during decompensated heart failure, mainly with inotrope dependence (6). In this context, short-term outcomes should have priority (7).
The dual blockade of the renin-angiotensin-aldosterone system with an ACE inhibitor and an AT1 angiotensin receptor blocker (ARB) used together has been demonstrated to be beneficial in heart failure patients (8,9). However, this dual inhibition has not been studied during heart failure decompensation or even when low cardiac output is present.
The objective of this study was to assess the effects of add-on therapy with angiotensin receptor blocker on plasma B-type natriuretic peptide (BNP) levels and hemodynamic measurements in heart failure patients with low cardiac output during hospitalization for decompensation.
METHODSThis study was carried out in the heart failure compensation unit of a tertiary cardiology center at a university hospital. Patients who are typically hospitalized in our medical facility are those with severe advanced heart failure in a decompensation period that does not respond to initial therapy; frequently, these patients present with low cardiac output (10). In our service, dobutamine is preferred over milrinone. It is not uncommon for some patients to experience dobutamine dependence and not be able to tolerate inotropic withdrawal.
PatientsThis was a randomized, double-blind, placebo-controlled clinical trial. The inclusion criteria were as follows: hospitalization for decompensated heart failure, defined by worsening of symptoms until fatigue or dyspnea at rest; low cardiac output, defined by the clinical-hemodynamic profile (4); dobutamine dependence; ejection fraction <0.45; spontaneous breathing; and receiving ACE inhibitors. The patients could have jugular ingurgitation, lower limb edema, ascites, and rales. Dobutamine dependence was defined by infusion for more than 15 days or an unsuccessful attempt at withdrawal. The exclusion criteria were as follows: serum creatinine >3.0 mg/dL, serum K>6.0 mEq/L, systolic blood pressure <70 mm Hg, aortic stenosis, and acute coronary syndrome in the previous 2 months. The patients were then assigned by permuted block randomization to 4 groups, stratified by sex, to losartan or placebo. The endpoints were changes in the BNP levels, cardiac index, pulmonary wedge capillary pressure, systemic vascular resistance, and successful withdrawal of dobutamine.
With a power of 80% and an estimated 45% reduction in BNP level between losartan and placebo, the calculated sample size was 18 patients. As a secondary endpoint, we estimated rates of successful withdrawal of 17% and 68% for patients in the placebo group and the ARB group, respectively, on the basis of our previous study (11).
Intervention proceduresPatients were allocated to the losartan or placebo group. Losartan or placebo was administered at 25 mg bid and increased to 50 mg bid 3 days later. The procedures were performed in a double-blind fashion for the first 7 days to control hemodynamic effects and for at least 3 more days until the attempt at dobutamine withdrawal. Captopril was the standardized ACE inhibitor used and was maintained unchanged during the double-blind period.
Hemodynamic monitoringThe patients underwent pulmonary artery catheterization, and cardiac output was obtained using the thermodilution technique (12). The hemodynamic data obtained included cardiac index, pulmonary artery pressure, pulmonary capillary wedge pressure, and right atrium pressure. Indexed systemic vascular resistance and indexed pulmonary vascular resistance were calculated. Hemodynamic measurements were performed at baseline and 7 days after the start of the intervention. The patients retained a percutaneous sheath introducer, and pulmonary artery catheters were used only for these two measurements.
BNP levels were measured using the automated immunoassay method at baseline and 7 days later. BNP serum levels were transformed by logarithmic correction. Subsequently, the changes incurred in the losartan and placebo groups were assessed by repeated-measures ANOVA.
Adverse eventsAdverse events included the occurrence of hypotension (systolic blood pressure below 70 mm Hg), hyperkalemia (K above 6.0 mEq/L), and worsening renal function (serum creatinine above 3.0 mg/dL). If the systolic blood pressure dropped below 70 mmHg, the daily dose of losartan or placebo was reduced by half. If this hypotension persisted, losartan or placebo was suspended.
Statistical analysisThe analysis was performed on an intention-to-treat basis and included all randomized patients. Continuous variables are expressed as the mean and standard deviation and were compared between the ARB and control groups by Student's t test. Categorical variables are expressed as numbers and proportions and were compared using the chi-square test or Fisher's test. Continuous variables at baseline and after the intervention were analyzed between groups using repeated measures ANOVA (13). P-values<0.05 (2-tailed) were considered significant.
Clinical interest variables or variables with p<0.100 by bivariate analysis underwent multivariate analysis by logistic regression (14) to calculate the odds ratios and respective 95% confidence intervals. Event free survival curves were constructed using the Kaplan-Meier method (15) and compared using the log-rank test (16). The mortality predictors were identified by Cox regression (17).
This study was approved by the research ethics committee of our institution and is registered with the Brazilian Health Ministry and with the Clinical Trials (www.clinicaltrials.gov; NCT 01857999). The patients or their guardians read and signed the informed consent form. This study was conducted according the principles of the Helsinki Declaration (2004).
RESULTSWe selected 21 patients between August 2008 and December 2010. The characteristics of the included patients were as follows: age 52.7 (SD = 11.5) years, ejection fraction 21.3% (SD = 5.8), dobutamine infusion 8.5 (SD = 3.8) mcg/kg.min, systolic blood pressure 98.5 (SD = 12.2) mmHg, pulmonary wedge capillary pressure 30.7 (SD = 7.7) mmHg, indexed systemic vascular resistance 1,918 (SD = 556) dynes.sec.cm-5.m-2, systemic vascular resistance 1,132 (SD = 371) dynes.sec.cm-5, cardiac index 2.8 (SD = 0.7) L/min.m2, and BNP 1,403 (SD = 950) pg/mL. There were 10 (47.6%) patients with Chagas disease. See Table1 for the complete baseline characteristics.
Baseline characteristics.
| Characteristic | Losartan (N = 10) | Placebo (N = 11) |
|---|---|---|
| Age (SD); years | 48.1 (12.7) | 55.7 (9.8) |
| Men (%) | 8 (80) | 8 (72.7) |
| Ejection fraction (SD); % | 20.4 (4.2) | 22.1 (6.9) |
| Dobutamine dose (SD); mcg.kg-1.min-1 | 7.2 (2.8) | 9.6 (4.3) |
| Cardiac index (SD); L.min-1.m-2 | 2.98 (0.36) | 2.64 (0.86) |
| Indexed systemic vascular resistance (SD); dynes.sec.cm-5.m-2 | 1,755 (294) | 2,052 (689) |
| Systemic vascular resistance (SD); dynes.sec.cm-5 | 1,022 (244) | 1,234 (445) |
| Pulmonary wedge capillary pressure (SD); mmHg | 30.0 (4.6) | 31.2 (9.8) |
| Captopril dose (SD); mg/d | 138.8 (35.6) | 129.6 (35.0) |
| Carvedilol (%) | 6 (60.0) | 9 (81.9) |
| Furosemide (%) | 9 (90) | 10 (91) |
| Sodium (SD); mEq/L | 133.2 (3.7) | 134.3 (4.3) |
| Potassium (SD); mEq/L | 4.23 (0.41) | 4.12 (0.43) |
| Urea (SD); mg/dL | 63.8 (35.8) | 59.4 (18.3) |
| Creatinine (SD); mg/dL | 1.21 (0.44) | 1.25 (0.30) |
| BNP; median (interquartile range); pg/mL | 1,259.5 (718.5 to 2,035.0) | 1,046.5 (604.5 to 2,061.0) |
| Hemoglobin (SD); g/dL | 13.4 (1.2) | 11.4 (1.0) |
SD: standard deviation; BNP: B-type natriuretic peptide.
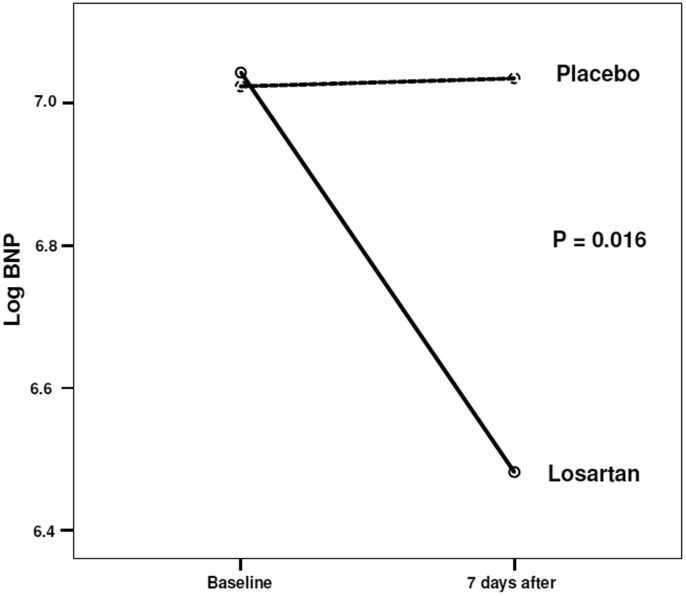
After 7 days, the BNP levels decreased (Figure1) by 37.4% in the losartan group and increased by 11.9% in the placebo group (mean difference -49.1%; 95% CI: -88.1 to -9.8%, p = 0.018).
There was no significant difference in the hemodynamic measurements. The systolic blood pressure decreased by 5.9% in the losartan group and by 3.2% in the placebo group (p = 0.796). The indexed systemic vascular resistance decreased by 7.7% in the losartan group vs. 10.5% in the placebo group (p = 0.280). The cardiac index increased by 6.7% and 6.9% in losartan and placebo groups, respectively (p = 0.203). The pulmonary capillary wedge pressure decreased by 22.9% and 5.1% in the losartan and placebo groups, respectively (p = 0.459). There were no complications related to the pulmonary artery catheter.
Successful dobutamine withdrawal occurred in 4/10 (40%) patients in the losartan group vs. 3/11 (27.3%) patients in the placebo group (p = 0.537 by logistic regression). The odds ratio was 1.78 (95% CI: 0.20 to 16.4) for successful dobutamine withdrawal in the losartan group.
The occurrence of adverse events was similar in both groups. A drop in systolic blood pressure below 70 mm Hg occurred in 3 (30%) patients in the losartan group and in 1 (9.1%) patient in the placebo group (p = 0.223); however, no persistent hypotension occurred. None of the patients in either group had hyperkalemia. Two patients (one patient from each group) had an increase in serum creatinine (>0.3 mg/dL) (p = 0.943). See Table2 for changes in the laboratory measurements.
Changes in variables.
| Variable | Losartan (N = 10) | Placebo (N = 11) | p-value |
|---|---|---|---|
| Potassium (SD); mEq/L; at baseline | 4.2 (0.4) | 4.1 (0.4) | |
| Potassium (SD); mEq/L; on 7th day | 4.3 (0.5) | 4.3 (0.4) | 0.959 |
| Creatinine (SD); mg/dL; at baseline | 1.21 (0.44) | 1.25 (0.30) | |
| Creatinine (SD); mg/dL; on 7th day | 1.15 (0.33) | 1.15 (0.29) | 0.911 |
| BNP; median (IQR); pg/mL; at baseline | 1,259.5 (1,318) | 1,046.5 (1,457) | |
| BNP; median (IQR); pg/mL; on 7th day | 895.5 (814) | 1,003.0 (1001.0) | 0.016 |
SD: standard deviation; BNP: B-type natriuretic peptide; IQR: interquartile range;
P-value from inter-group comparison.
After a follow-up of 465 days (95% CI: 317 to 613), 10 patients died (5 in each group) (p = 0.640), as shown in Figure2. Cox regression identified heart failure with ischemic etiology as an independent predictor of all-cause mortality, with a relative risk of 14.3 (95% confidence interval: 1.6 to 130.0, p = 0.019).
DISCUSSIONThe main finding of this study was the decline in BNP levels with angiotensin-receptor blocker add-on therapy in patients with acute decompensated heart failure and a low cardiac output state. Systemic vascular resistance, cardiac index, and pulmonary wedge capillary pressure remained unchanged, as did the clinical endpoint. These findings are based on 42 hemodynamic and BNP measurements in 21 patients.
The novelty of the present study lies in the demonstration of a more intense reduction in BNP levels (37.4%) with add-on ARB therapy during decompensation (compared with 25% in stable outpatients). Moreover, this effect was achieved in a 7-day period, whereas a 22-week period was reported in a previous study (18). In another study with stable class II-III heart failure patients, candesartan add-on therapy reduced amino-terminal proBNP by 20% in 6 months (19). Different from stable heart failure patients, the management of patients with decompensation requires specific strategies. For this reason, add-on ARB therapy could be more important during heart failure decompensation, when BNP levels and neuro-hormonal activation are higher (4).
A generally low cardiac output state during decompensation of heart failure is often neglected in randomized clinical trials. Moreover, patients with unsuccessful withdrawal of inotropic therapy have mortality rates higher than 79% (20). Our findings could be particularly important for inotrope-dependent heart failure patients who are not able to receive a heart transplant. For these patients, the effects of drug therapy should be faster and more intensive than for patients with mild disease.
In addition, the lower ejection fraction, the low cardiac output, the higher systemic vascular resistance, and the higher BNP levels demonstrated the severity of heart failure in our patients.
Hemodynamic condition during heart failure decompensationWe found high systemic vascular resistance and high pulmonary capillary wedge pressure in our patients during acute heart failure decompensation. The high systemic vascular resistance occurred despite the fact that the patients were taking a high dose of an ACE inhibitor (captopril: 134 mg/d). In fact, Parker et al. observed a reduction in systemic vascular resistance in patients with severe heart failure who were taking ACE inhibitors; however, this resistance remained above the normal values (21,22). The cardiac index was normal; however, all patients were using intravenous inotrope. Our findings are similar to those of the Escape trial, which justifies the use of multiple vasodilators with add-on ARB therapy. Interestingly, hemodynamic improvement in the placebo group occurred even though all medications remained unchanged. Non-pharmacological factors could be involved in this finding, such as rest, salt restriction, physiotherapy, and the “inertial” effect of drug therapy before the hemodynamic measurements.
Dobutamine is indicated for low cardiac output in severe heart failure based on International Guidelines; however, a meta-analysis describing concerns regarding the increase in mortality has been published (23). Nevertheless, this meta-analysis included outpatients who received intermittent therapy. Despite these concerns, in practice, physicians continue to prescribe dobutamine to 22.3% of patients who are hospitalized for heart failure (24,25). Consequently, our results showed that add-on ARB therapy is an alternative to this high-risk situation (i.e., low cardiac state and inotrope dependence).
ARB-ACE inhibitor association pathophysiologyThe renin-angiotensin-aldosterone system plays an important role in the pathophysiology of heart failure. In systolic heart failure, activation of this system occurs, which worsens cardiac remodeling and hemodynamic conditions, creating a vicious cycle. The blockade of this system with an ACE inhibitor and an aldosterone antagonist reduces morbidity and mortality.In addition, the renin-angiotensin-aldosterone system interacts with the cardiac peptide system. Plasma BNP levels increase in heart failure due to BNP release by myocytes after distension. BNP has diagnostic and prognostic values in heart failure (26). Used as a guide for heart failure treatment, BNP reduces death, hospital admission, and decompensation, most likely through higher doses of diuretics, ACE inhibitors, and spironolactone (27,28,29). However, the use of biomarkers such as BNP as a surrogate endpoint remains controversial.
The ARB-ACE inhibitor association has been proven to be beneficial in reducing clinical events such as cardiovascular mortality and hospitalization (8,9). In our study, we applied the tactic of ARB add-on therapy in patients with more severe heart failure during decompensation and low cardiac output, and we found a hormonal benefit. The survival analysis did not identify a difference between the losartan and placebo groups; however, the procedures were performed in a double-blind fashion for 7 days, and it is very unlikely that a short-term intervention could have an influence on late outcomes.
The HEAAL study demonstrated that a high dose of losartan reduces the endpoint rate compared with a low dose (30). The occurrence of hyperkalemia was 2.79% in the high-dose group and 1.87% in the low-dose group (p<0.001); creatinine was also increased in the high-dose group (7.12%) compared with the low-dose group (4.73%) (p<0.001).
This association has been demonstrated to be beneficial for patients on hemodialysis, leading to a significant reduction in mortality and hospitalization (31). Adverse effects—mainly hypotension—occurred in 16.3% of patients in the ARB group versus 10.7% of patients in the placebo group.
Safety of the ARB-ACE inhibitor associationSafety is another concern related to the ARB-ACE inhibitor association. In the present study, the ARB-ACE inhibitor association did not increase the occurrence of adverse effects in the short-term. Use of this association for treatment of hypertension did not reduce cardiovascular events; rather, it increased adverse effects (32). On the other hand, in heart failure patients, this association decreased mortality and hospitalization with an increase of approximately 5% in adverse effects (8,9). In addition, a meta-analysis (33) revealed a 2.3% increase in the risk of developing an adverse event. However, patients with more advanced heart failure are the minority in these studies. The occurrence of adverse events could be more frequent in patients with more severe disease, although the benefit could be greater. Moreover, worsening renal function has special importance because it has been related to a worse prognosis in severe heart failure (34).
Study limitationsThe sample size of our study was small; thus, the analysis of clinical events was somewhat challenging. Our primary endpoint was a change in BNP, which is a surrogate endpoint for clinical events. It is possible that the high percentage of Chagas disease cardiomyopathy interfered with our results because these patients have attenuated vasoconstriction; consequently, multiple vasodilation strategies were not as effective as we expected.
In summary, short-term add-on therapy with losartan reduced BNP levels in patients hospitalized for decompensated severe heart failure and low cardiac output with inotrope dependence. A non-significant hemodynamic improvement and an increase in the probability of successful dobutamine withdrawal were observed.
ACKNOWLEDGMENTSThis study was sponsored by the São Paulo Research Foundation (FAPESP - grant number 2006/06463-9).
AUTHOR CONTRIBUTIONSOchiai ME conceived the study and performed patient selection, invasive monitoring, and manuscript writing. Brancalhão EC and Puig RS performed patient selection, invasive monitoring, and data analysis. Vieira KR reviewed the literature and performed patient selection as well as invasive monitoring. Cardoso JN performed patient selection and invasive monitoring and provided helpful suggestions. Oliveira-Jr MT assisted with manuscript writing. Barretto AC assisted with conception of the study and manuscript writing.
No potential conflict of interest was reported.