Prolonged tissue ischemia leads to changes in microcirculation and production of oxygen free radicals. The event eventually responsible for tissue death is the no-reflow phenomenon and its management is a challenge for the surgeon dealing with replantation or transplantation. We introduce a model of warm ischemia and reperfusion of the lower limb of rats with which we studied the effect of allopurinol and streptokinase.
METHODSection of the lower limb with preservation of vessels and nerves was performed in 110 rats. Femoral vessels clamped for periods of 0, 2, 4, 6, and 8 hours of ischemia were allowed to reperfuse (groups M0, M2, M4, M6, and M8 respectively). Other groups, E1, E2, and E3, received streptokinase, allopurinol, or a combination of the two drugs after 6 hours of ischemia.
RESULTSViability rates of the ischemic limbs after 7 days were 100% (M0), 80% (M2), 63.6% (M4), 50% (M6), and 20% (M8). In the experimental groups, E1, E2, and E3, viability rates were 67% (E1), 70% (E2), and 70% (E3). Groups M0, M2, M4, M6, and M8 differed among themselves except for groups M4 and M6. Group E1 had a higher rate of limb viability than M6 (control group) but not than M4. Groups E1, E2 and E3 had higher rates of limb viability than M6 but not than M2 or M4.
DISCUSSIONThe results suggest that increased viability of limbs after 6 hours of ischemia occurs when allopurinol or streptokinase is used. The combination of the two drugs does not appear to produce any additional effect.
A isquemia prolongada dos tecidos leva a alterações na microcirculação e liberação de radicais livres do oxigênio, evento que pode resultar na morte do tecido, conhecido como fenômeno de não reperfusão. Um modelo em ratos de isquemia quente e reperfusão do membro posterior é proposto, e os efeitos dos fármacos alopurinol e estreptoquinase foram estudados.
MÉTODOSSecção do membro posterior com preservação dos vasos e nervos foi realizada em 110 ratos. O pinçamento vascular e posterior reperfusão após isquemia quente de 0, 2, 4, 6 e 8 horas formou os grupos M0, M2, M4, M6 e M8 respectivamente. Outros grupos E1, E2 e E3 receberam, respectivamente, alopurinol, estreptoquinase e a combinação de ambas as drogas, após seis horas de isquemia.
RESULTADOSAs taxas de viabilidade dos membros isquêmicos, observadas após sete dias foram: M0 - 100%, M2 - 80%, 4 - 64%, 6 - 50% e M8 - 20%. As taxas de viabilidade dos grupos experimentais foram 67%(E1), 70%(E2) e 70%(E3). Os grupos M0, M2, M4, M6 e M8 foram diferentes entre si exceto os grupos M4 e M6. E1, E2 e E3 resultaram em viabilidade de membros maiores que M6(controle), mas não em relação ao M2 e M4.
DISCUSSÃOOs resultados sugerem um aumento da viabilidade de membros após 6 horas de isquemia quando utilizado os fármacos alopurinol ou estreptoquinase. A associação de estreptoquinase e alopurinol não mostrou efeito adicional.
Replantation of extremities after amputation or major trauma is not always successful, and the most important factor is the time elapsed from the beginning of ischemia. Viability and final functional result depend on the prevention of damaging effects of anoxia. The involved tissues have different resistances to anoxia. Skeletal muscle is the least tolerant to ischemia and the anatomical, metabolic, and pathophysiological effects having been thoroughly studied.1–3 Maintenance of muscle viability is critical for the success of replantation.
The effort to improve limb viability has led authors to introduce different models to study ischemic limb perfusion and the effect of different drugs on survival rates.4 No consensus exists regarding the best model for studying ischemia, and limb survival rates differ from model to model. Successful replantations with longer periods of ischemia have been reported.1
Although microsurgical anastomosis is critical, the distal capillary circulation is equally important in terms of the viability of microsurgical flaps and replanted limbs. Prolonged ischemia followed by reperfusion produces irreversible damage to microcirculation, leading to obstruction of blood flow to peripheral tissues. These alterations to microcirculation constitute the no-reflow phenomenon.3,5–9 Obstruction is progressive, with thrombus formation in the microcirculation, platelet aggregation, and tissue edema. Irreversible damage and loss of limb or of microsurgical flap can result.2,8,10,11
The possible role of oxygen free radicals in the pathogenesis of tissue lesions after reperfusion has been studied, as has the use of pharmacological agents to prevent this phenomenon.12,13 Encouraging results have been obtained using antioxidants, as well as a number of other drugs and agents in different tissues.5,14,15 The superoxide radical is created in all aerobic organisms under various conditions and causes peroxidation of membranes and proteins resulting in tissue destruction and irreversible cell injury. 2,7,16,17
Vascular thrombosis is a significant complication in microsurgery. There are several causes for thrombosis, all of which may lead to failure of a microvascular anastomosis.18,19 Some factors are inherent to replanted tissues, while others depend on surgical technique. Tissue trauma has been recognized as the main cause of limb loss.20 Various anti-thrombotic and anti-inflammatory drugs have been studied in experimental flaps in trying to prevent vascular thrombosis.19,21
An animal model using rats was developed in the Laboratory for Experimental Microsurgery at the Fac. Med. Univ. São Paulo to determine the maximum ischemic period tissues can sustain and the influence of different drugs on the prevention of deleterious effects of warm ischemia and reperfusion. This paper presents this experimental model of warm ischemia and reperfusion and its use in assessing the effect of allopurinol and streptokinase on the survival of the extremities.
METHODThis project was approved by the Institutional Ethics Committee, and follows the NIH guidelines for animal experiments.
One hundred and ten male Wistar rats ranging in age from 16 to 20 weeks and weighing between 290 and 400 g were used. The animals were maintained in individual cages with food and water ad libitum, a light/dark period of 12/12 hours, and a room temperature of 23°C. The same surgeon performed all surgical procedures.
Surgical procedureThe animals were anesthetized using intraperitoneal sodium pentobarbital (50 mg/kg) injection. A subtotal amputation of the right posterior limb was accomplished, preserving the femoral vessels and the sciatic nerve.
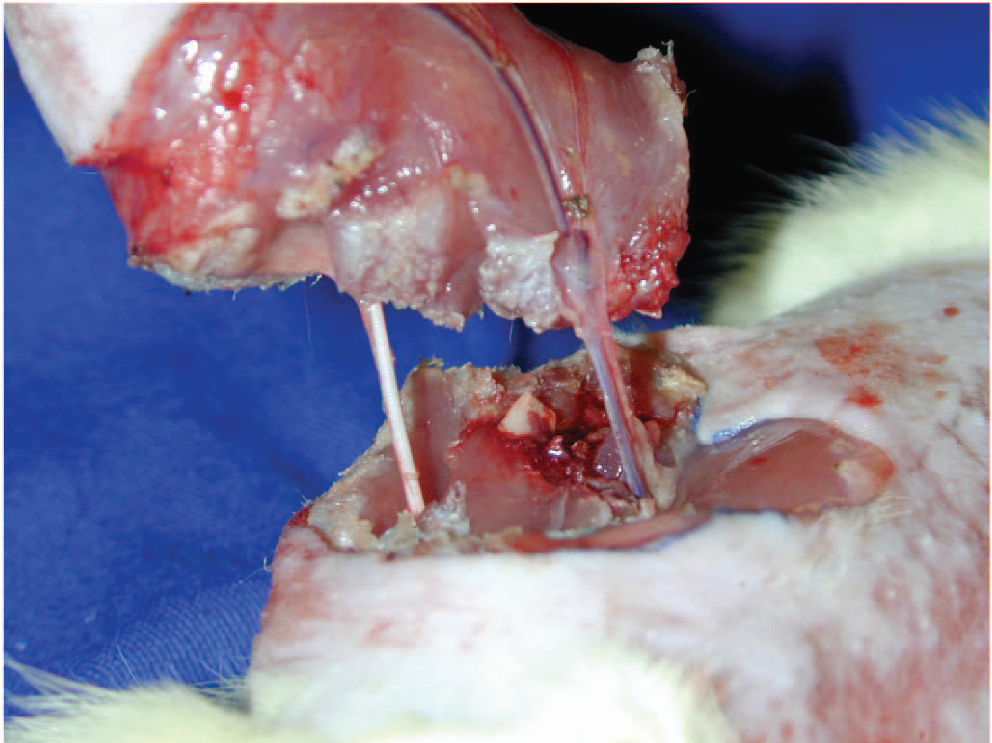
A circular incision in the mid-portion of the thigh was performed around the entire circumference including skin and subcutaneous tissue. Femoral vessels and nerve were dissected using a surgical microscope (Karl Kaps model D-35614 ASSLAR - 16X magnification), and muscles of the mid-third thigh were severed, but the sciatic nerve was identified and kept intact. Osteotomy of the mid-third of the femur was performed with a manual saw (Fig. 1). Those tissues were immediately reconstructed, starting with the bone, by intramedullary fixation of the femur with a 2 cm needle segment and instant cyanoacrylate ester glue. Horizontal U stitches of 5-0 nylon were used for the muscle synthesis.
An atraumatic microvascular clamp was applied to right femoral vessels before the emergence of the superficial caudal epigastric vessels, avoiding the inclusion of the femoral nerve (Fig. 2). A negative milking test certified interruption of arterial and venous flow, and ischemia was considered to be complete.
Fluid volume was replaced postoperatively by a subcutaneous injection of 50 mL/kg of saline. The mean time for subtotal amputation and replantation was 33 minutes (range, 15-45 minutes). Twenty-one animals died during surgery or within 7 postoperative days, a perioperative mortality rate of 19%. This mortality rate was similar to that experienced by Ferreira et al.1 (25.5%) and Shapiro22 et al. (15.4%).
Period of warm ischemiaThe animals were submitted to different times of warm ischemia after the vessels were clamped. During this period they were allowed to recover in individual cages at a room temperature of 25°C, achieved using a 45 W and 127 V halogen lamp that was maintained 30 cm from the cage.
ReperfusionAt the end of the ischemic period, rats were reanesthetized. Dissection of the left femoral vessels and their branches was performed, a needle (29G) was inserted in the superficial caudal epigastric vein, 1 mL of saline, streptokinase, allopurinol, or streptokinase/alopurinol was injected, and the vascular clamp was removed.
Patency of the femoral vessels was confirmed by a positive milking test and absence of thrombi. The presence of thrombi and absence of flow in femoral vessels excluded the animal from the experiment.
GroupsThe animals were divided into 5 model groups with 10 to 12 rats each that were submitted to ischemic periods of 0, 2, 4, 6, and 8 hours (M0, M2, M4, M6, and M8, respectively). It was determined that 6 hours of warm ischemia (M6) would be used as the control for the experimental groups. Three experimental groups with 10 or 12 animals per group were submitted to 6 hours of ischemia and received intravenous solutions of streptokinase, allopurinol or streptokinase/allopurinol (E1, E2, and E3, respectively) after 6 hours and immediately before the vascular clamp was removed (Table 1).
Allopurinol was diluted in 1N sodium hydroxide until it fully dissolved. Hydrochloric acid (1N) was added to this alkaline solution until the start of allopurinol precipitation, indicating the minimum pH for solubility. A final drop of 1N sodium hydroxide was used to redissolve the precipitate, and water was added to obtain the necessary final volume. One mL of the resulting solution contained 45 mg/kg of allopurinol (Allopurinolâ—Sigma Aldrich).
The streptokinase solution used contained 20000 IU/kg of streptokinase (Streptaseâ 1500000 IU—Hoeschst Marion Roussel) diluted in 1 ml of 0.9% saline.
Analysis of limb viabilityThe animals were observed daily over a 7-day period. Limb viability was determined by clinical examination as an all-or-nothing response. A nonviable limb was defined by total limb necrosis. A viable limb was defined by total limb survival or total survival with partial necrosis of the skin near the suture with viable underlying muscle and normal hair growth.
Results were converted into percentage of limb viability per group. The groups were compared among themselves and analyzed using the chi-square test. The level of significance was set at 5% (P < .05).
RESULTSGroupsGroup M0 was submitted to 0 hours of ischemia and displayed a 100% limb viability rate (LVR). The LVR of the groups progressed as follows: M2, 80%; M4, 64%; M6, 50%; and M8, 20%. Differences between groups M0 vs. M2, M2 vs. M4, and M6 vs. M8 were statistically significant. The difference between groups M4 vs. M6 was not statistically significant.
After 6 hours of ischemia, group E1 (streptokinase) displayed an LVR of 67%, which was statistically different from that of group M6 (6 hours and no drug) (P < .05). No statistical difference was observed in the VLR rate between groups E1 and M4 (4 hours and no drug).
Group E2 (allopurinol) progressed with an LVR of 70% that was statistically different from that of group M6 (P < .05), but not from M2 or M4.
The association of allopurinol and streptokinase (E3) had an LVR of 70% and was statistically different from that of M6 (P < .05). No statistical difference was observed between E3 and M2 or M4.
Differences among groups E1, E2, and E3 were not statistically significant.
Fig. 3 and 4 (graphs)
A variety of causes have been proposed for the no-reflow phenomenon. Ames et al.,7 in 1968, first described a no-reflow phenomenon in areas of ischemic brain. Miller et al.23 observed that arteriovenous shuntings occurred in replanted limbs, and this could also reduce blood flow. May et al.6 mentioned that stasis and loss of intravascular fluid with consequent exposure of subintimal collagen could lead to thrombosis after replantation. Urbaniak et al.24 classified the multiple factors responsible for the no-reflow phenomenon into 3 groups: ischemia, injury to the vascular intima, and systemic and local responses. This triad leads to histological and metabolic changes in the microcirculation that include vasoconstriction of arterioles, endothelial edema, regional blood stasis, adhesion and migration of leukocytes, focal hemorrhage, platelet aggregation, production of oxygen free radicals, and acidosis.
Several experimental models have been developed to study this phenomenon. Stock et al.25 observed irreversible metabolic changes after 4 hours of ischemia of rat hind limbs. Swartz et al.26 reported 100% necrosis of rat hind limbs submitted to 6 hours of ischemia.
Zdeblick et al.27 studied replanted posterior rat limbs after several hours of ischemia and observed that limb viability exhibited an all-or-nothing response. After 2 or 3 hours of ischemia, viability rates were 100%, after 4 hours the viability rate dropped to 50%, and after 6 hours, 20%. Edwards et al.28 also observed an all-or-nothing response in survival of rat hind limbs after replantation. The limbs were submitted to warm ischemia for 5 hours and the resulting LVR was 50%. Concannon et al.29 obtained an LVR of 30% in posterior rat limbs submitted to 6 hours of warm ischemia and also observed an all-or-nothing response. Olivas et al.30 studied ischemia of the rat’s cremaster muscle and observed that 7 or 8 hours of ischemia is the critical time for reversibility of reperfusion injuries in muscles.
The operative procedure in the present study consisted of subtotal hind limb amputation, keeping intact the vascular pedicle and nerve, a model which is similar to that used by Concannon et al.29 Keeping the vascular pedicle intact avoided the need for microvascular anastomosis and a new variable to be controled.22 The intact sciatic nerve prevented loss of sensibility and limb autophagia. which was only observed after the fourth postoperative day when there was necrosis of the limb. Concannon et al.29 described autophagia in viable and nonviable limbs with nerve injury.
Body fluid volume was replaced with 50 mL/kg of saline. Blood loss via bone marrow in intramedullary fixation was reported as cause of death by Ferreira et al.1 Instant glue was used in this study for bone synthesis so as to avoid blood loss through the bone marrow.
In order to determine the critical time of ischemia, 5 groups were compared, ranging from immediate reperfusion to 8 hours, and in accordance with reported data, and an all-or-nothing response was recorded. The results evidenced a negative correlation between ischemic time and viability, therefore conferring reliability to the ischemia model.
Effect of streptokinaseMicrovascular thrombosis is an important component of the no-reflow phenomenon. Blood stasis, endothelial ischemic injury, and exposure of the subendothelium activate the coagulation cascade and lead to formation of fibrin and platelet aggregation. Fibrinolytic drugs have been used in microsurgery as prophylaxis for preventing thrombosis of anastomosis. Urokinase, heparin, and prostaglandin E1 were part of the replantation protocol of Yamano31 and of Fukui & Tamai.32 Vertos & Tsavissis18 have used streptokinase and low molecular weight dextran routinely after replants. Fibrinolytic drugs can also be employed therapeutically to reduce the effects of ischemia injury. Their effectiveness has been shown in clinical treatment of various thrombotic pathologies, such as acute coronary arterial thrombosis, deep venous thrombosis, and pulmonary thromboembolism.
The role of thrombolytic agents in preserving viability in microsurgical flaps and replants is not fully established. A number of drugs have been tested with controversial results. More recently, streptokinase and rt-PA have been successfully used in replants and in experimental models of thrombosis.19
In this study, group E1 received an intravenous dose of 20,000 IU/kg of streptokinase after 6 hours of warm ischemia. This dose was based on those used clinically for systemic fibrinolysis and was also used by Busnardo et al.19 to study thrombosis in microsurgical anastomosis.
The LVR after streptokinase (67 %) was better and significantly different from the control group M6 (50%). This result suggests that limbs submitted to 6 hours of ischemia and treated with streptokinase were protected compared to controls.
Effect of allopurinolThere are several references regarding the benefits of allopurinol and other antioxidants in ischemic models in fasciocutaneous flaps in rats and pigs.5,9,14,29
In this study, group E2 underwent limb ischemia for 6 hours and was treated with 45 mg/kg of intravenous allopurinol. Concannon et al.29 also used this dose and administration route.
The LVR of the allopurinol group (70%) was significantly higher than that of group M6 (50%). This data suggests that 6-hour ischemic limbs treated with allopurinol were protected compared to controls.
No statistical difference was found between groups E2 (70%) and E3 (70%), implying that there was no additional benefit from using a combined streptokinase-allopurinol treatment.
These data are similar to results found by Concannon et al.,29 in which a viability rate of 60% was observed in 6-hour ischemic rat limbs after administration of allopurinol. Ferrari et al.33 noticed a reduction in compartmental pressure of amputated rat limbs with various ischemic times and administration of allopurinol.
In summary, the proposed rat hind limb experimental model is simple, and it has a low rate of complications and mortality. It is reliable and suitable for studying ischemia, reperfusion, and the no-reflow phenomenon with an all-or-nothing response.
This report demonstrated that viability rates of limbs after 6 hours of ischemia increased from 50% to 64% with streptokinase administration and to 70% with allopurinol. No additional effect was obtained with the combination of these drugs.
In summary, the proposed rat hind limb experimental model is simple, and it has a low rate of complications and mortality. It is reliable and suitable for studying ischemia, reperfusion, and the no-reflow phenomenon with an all-or-nothing response.
This report demonstrated that viability rates of limbs after 6 hours of ischemia increased from 50% to 64% with streptokinase administration and to 70% with allopurinol. No additional effect was obtained with the combination of these drugs.