The aim of this study is to determine clinical, pathological, and treatment-relevant variables associated with long-term (90-month) overall survival in patients with lung metastases undergoing pulmonary metastasectomy.
METHODS:A retrospective review was performed of patients who were admitted with lung metastases, and who underwent thoracotomy for resection, after treatment of a primary tumor. Data were collected regarding demographics, tumor features, treatment, and outcome.
RESULTS:Patients (n = 529) were submitted to a total of 776 thoracotomies. Median follow-up time across all patients was 21.6 months (range: 0–192 months). The postoperative complication rate was 9.3%, and the 30-day mortality rate was 0.2%. The ninety-month overall survival rate for all patients was 30.4%. Multivariate analysis identified the number of pulmonary nodules detected on preoperative CT-scan, the number of malignant nodules resected, and complete resection as the independent prognostic factors for overall survival.
CONCLUSION:These results confirm that lung metastasectomy is a safe and potentially curative procedure for patients with treated primary tumors. A select group of patients can achieve long-term survival after resection.
The presence of metastases in patients with malignant tumors is a sign of advanced systemic disease1. Consequently, few patients survive more than one year after diagnosis.2 However, over 20% of patients with solid tumors, including colorectal adenocarcinoma, may have metastases exclusively in the pulmonary parenchyma, with no detectable involvement of other organs. In these patients, surgical resection of lung metastases may significantly impact long-term survival.3 We have recently reported on metastatic lung cancer in Brazil4 and reviewed possible sources of new anti-tumoral drugs from Brazilian plant extracts.5
Pulmonary metastasectomy is currently indicated for patients with the following criteria: primary tumor controlled; no evidence of metastases outside lung parenchyma; possibility of complete resection as verified by chest imaging; pulmonary function compatible with the proposed lung resection procedure; and lack of another available treatment that would be more effective than metastasectomy.6 Using these criteria for metastasectomy, several retrospective series have reported five-year survival rates ranging from 30% to 40%.7
These results are encouraging considering that all of the patients included presented with metastatic disease. However, there is a clear need to refine these criteria to better identify patients who would benefit from surgical resection of lung metastases. More accurate criteria may eventually improve long-term survival rates and avoid unnecessary surgical procedures. In order to define the selection factors, many authors have reviewed their experience with lung metastasectomy in an effort to identify variables associated with better survival. The majority of these series included a limited number of patients treated over a long period of time, and the heterogeneity of the studied population did not allow for definitive conclusions. Today, complete surgical resection, long disease-free intervals, and a suitably low number of metastases are considered the most important variables associated with better survival rates.8
Since the early 1990s, our institution has employed a continuous protocol-oriented approach to treat patients with resectable lung metastases. Our goal was to define factors related to outcome following lung metastasectomy in our patient population. All patients in the studied population underwent similar preoperative imaging, together with clinical and surgical evaluations. This was done to generate a more homogeneous and comparable group of patients, each of whom had detailed information registered in their individual records.
The goal of this study is to evaluate outcomes following the surgical treatment of lung metastases in patients treated at a single institution, as well as to identify prognostic factors that may significantly impact long-term overall survival. Our results will allow us to more precisely define the subgroups of patients who may benefit from surgical therapy.
METHODSThis is a retrospective analysis of patients previously diagnosed with primary malignant solid tumors and who subsequently underwent surgical resection of lung nodules in the context of suspected or diagnosed metastatic lesions. All patients were admitted and treated in the Department of Thoracic Surgery of AC Camargo Hospital from 1990 to 2006. This study was approved by the ethics committee at our institution.
Data were collected from medical record reviews of individual patients. The following characteristics of the primary tumor were recorded: primary site, date of surgical resection, histological type, and adjuvant or neoadjuvant treatment associated with the resection.
All patients were evaluated for lung nodules during the pretreatment evaluation and staging of a neoplasm or during routine follow-up after the treatment of various primary tumors. Disease-free interval was defined as the time period between the treatment of the primary tumor and the diagnosis of pulmonary metastases.
Patients were considered eligible for pulmonary metastasectomy if they presented with the following characteristics: 1) primary tumor controlled or controllable, 2) nodules confined to the lung parenchyma, 3) nodules that were amenable to surgical resection, 4) pulmonary function and clinical condition that were compatible with the planned operation, 5) predictable remaining lung function after resection that would allow for adequate postoperative quality of life, and 6) non-availability of a more suitable treatment option for the metastases.
All patients underwent a chest CT scan in order to evaluate the resectability of their pulmonary nodules. Radiology reports were reviewed in each patient file, and the following characteristics were recorded: number of pulmonary nodules, size of the largest nodule, and laterality. The preoperative evaluation of the nodules was routinely performed by the radiologist and the surgeon. In cases of significant discrepancies between the number of nodules identified by the surgeon and by the radiologist, the evidence was discussed with a specialist. The number of nodules included in this retrospective study was the final number confirmed by the surgeon after complete preoperative evaluation.
In the case of bilateral nodules on admission, a staged thoracotomy was performed starting on the side with the lowest likelihood of a complete resection. Several factors determined which side to start with, including the number of nodules identified by chest CT, the anatomical position of one or more nodules relative to the pulmonary hilum, and the potential need for a more complex and extensive lung resection.
Patients were placed under general anesthesia with selective single lung ventilation. Surgical access to the thorax was performed by lateral thoracotomy even for patients with bilateral metastases. In all cases, we attempted to completely resect all lung nodules, preserving lung parenchyma by resecting the tumor with a small margin (5 mm to 10 mm). We did not routinely perform mediastinal lymph node dissection or sampling. In the presence of a single pulmonary nodule, frozen section analysis was mandatory. If histological evidence suggested a possible primary lung tumor, the patient underwent pulmonary lobectomy with radical mediastinal lymph-node dissection when possible. Lung nodules were identified individually according to the site of resection and sent for histopathological analysis.
Date of surgery, type of resection (complete or incomplete), number of malignant and benign resected nodules, size of the largest nodule, and type of lung resection (wedge, segmentectomy, lobectomy or pneumonectomy) were registered according to the surgical report in individual patient records.
Some patients received chemotherapy, based on the medical oncologist’s opinion, before or after a surgical resection of lung nodules. The type of treatment and radiological response (complete, partial, stable or progression of disease, as defined by RECIST9) were collected.
Patients were discharged from the hospital following chest tube removal. Postoperative complications included: fever>38°C, bleeding, air leakage for more than three days, Intensive Care Unit stay for more than two days, cardiac complications (arrhythmia, ischemia, infarction), pulmonary atelectasis, embolism or respiratory failure, renal failure, neurological complications (coma, stroke, motor and sensitive dysfunctions), and infection (bacterioscopy or culture confirming the presence of a bacterial infection).
Beyond hospital discharge, patients were followed-up by clinical examination, with radiological evaluations (chest X-rays and CT scans) every three months during the first two years post-resection and every six months subsequently until the fifth year. Annual radiological follow-up was performed thereafter. Other ancillary tests were performed consistent with symptoms or clinical suspicion of recurrence in organs other than the lungs. We recorded recurrence when new lesions were identified in the lungs or in other organs. When necessary, we performed a histological confirmation of a new tumor. When recurrence was again limited to the lung parenchyma, and if the disease was considered resectable by the attending thoracic surgeon, the patient underwent additional metastasectomies as needed.
After complete surgical resection, all patients were evaluated in the department of clinical oncology, and systemic treatment was administered by the clinical oncologist.
The characteristics of the patients included in this study are shown in Table 1.
Patient characteristics
| Variables | n | % |
|---|---|---|
| Age (mean: 45.7 years, median: 50.1, range: 3.7–89.2 years) | ||
| Age subgroups (years) | ||
| 0–12 | n=38 | 7.2% |
| 13–40 | n=155 | 29.3% |
| 65 | n=231 | 43.7% |
| 70 | n=105 | 19.8% |
| Gender | ||
| Male (n=267) | 50% | |
| Female (n=262) | 50% | |
| Primary tumor site | ||
| Bone | n=105 | 19.9% |
| Head-Neck | n=101 | 19.2% |
| Soft tissue | n=72 | 13.7% |
| Breast | n=69 | 13.1% |
| Colorectal | n=51 | 9.7% |
| Skin | n=40 | 7.8% |
| Uterus | n=18 | 3.4% |
| Kidney | n=15 | 2.8% |
| Stomach | n=8 | 1.5% |
| Bladder | n=3 | 0.6% |
| Other | n=44 | 8.4% |
| Histology of the primary tumor | ||
| Adenocarcinoma | n=154 | 29.2% |
| Osteosarcoma | n=86 | 16.3% |
| Squamous cell | n=81 | 15.3% |
| Soft tissue sarcoma | n=75 | 14.2% |
| Malignant melanoma | n=48 | 9.1% |
| Other | n=84 | 15.9% |
| Disease-free interval (months) | ||
| ≤ 12 | n=227 | 42.9% |
| >12 | n=302 | 57.1% |
| Number of nodules on CT scan | ||
| ≤ 2 | n=301 | 56.9% |
| 3–4 | n=79 | 14.9% |
| >4 | n=149 | 28.2% |
| Laterality of the nodules | ||
| Right lung | n=185 | 35% |
| Left lung | n=123 | 23.3% |
| Bilateral | n=220 | 41.7% |
| Type of resection | ||
| Pneumonectomy | n=3 | 0.6% |
| Lobectomy | n=65 | 12.3% |
| Segmentectomy | n=180 | 34% |
| Wedge resection | n=260 | 49.1% |
| Biopsy | n=20 | 3.8% |
| Number of resected nodules: median 1 (0-multiple) | ||
| Number of malignant nodules resected | ||
| <2 | n=180 | 44.6% |
| 2–4 | n=164 | 40.6% |
| >4 | n=60 | 14.8% |
| Number unknown | n=125 | |
| Diameter of greater nodule | ||
| ≤1 cm | n=163 | 31% |
| 1.1–3 cm | n=256 | 48.7% |
| >3 cm | n=107 | 20.3% |
| Number of thoracotomies | ||
| 1 | n=331 | 62.6% |
| 2 | n=134 | 25.3% |
| 3 | n=40 | 7.6% |
| >3 | n=24 | 4.5% |
| Complete resection at last thoracotomy | ||
| Yes | n=408 | 77.1% |
| No | n=120 | 22.7% |
| Adjuvant chemotherapy after lung resection | ||
| No | n=354 | 66.9% |
| Yes | n=160 | 30.2% |
| Unknown | n=15 | 2.8% |
Continuous data are presented as medians, and categorical data are presented as percentages. Overall and disease-free survival rates were estimated using Kaplan-Meier analysis10. Log-rank and Breslow analyses were used to compare differences between variables. Overall survival was estimated from the date of surgery to the date of last follow-up or until death from any cause. The influence of each variable on overall and disease-free survival was calculated.
Multivariate analysis to determine the independent prognostic factors for overall survival was determined by the Cox proportional hazard model.11 Whenever more than one procedure was performed, survival time was determined, with time zero denoting the first thoracotomy.
Statistical analyses were performed using SPSS 11.5 for Windows. Significant differences were defined as p<0.05.
RESULTSThe patients included in this retrospective study (n = 529) underwent a total of 776 thoracotomies. The average follow-up time for all patients was 40 months, with a median of 21.6 months (range: 0–192 months), and eight patients (1.5%) were lost to follow up. Most patients were discharged by the fifth postoperative day (length of stay in hospital ranged from two days to 27 days, with a median of four days). The postoperative complication rate was 9.3% for the 776 thoracotomies. More than one complication occurred in some patients (infection n=19, atelectasis n=29, cardiac arrhythmia n=18, stroke n=2, myocardial infarction n=3, prolonged air leak n=28), and the 30-day mortality rate was 0.2% (n=2, due to respiratory failure and stroke).
The ninety-month overall survival rate for all patients was 30.4% (Figure 1).
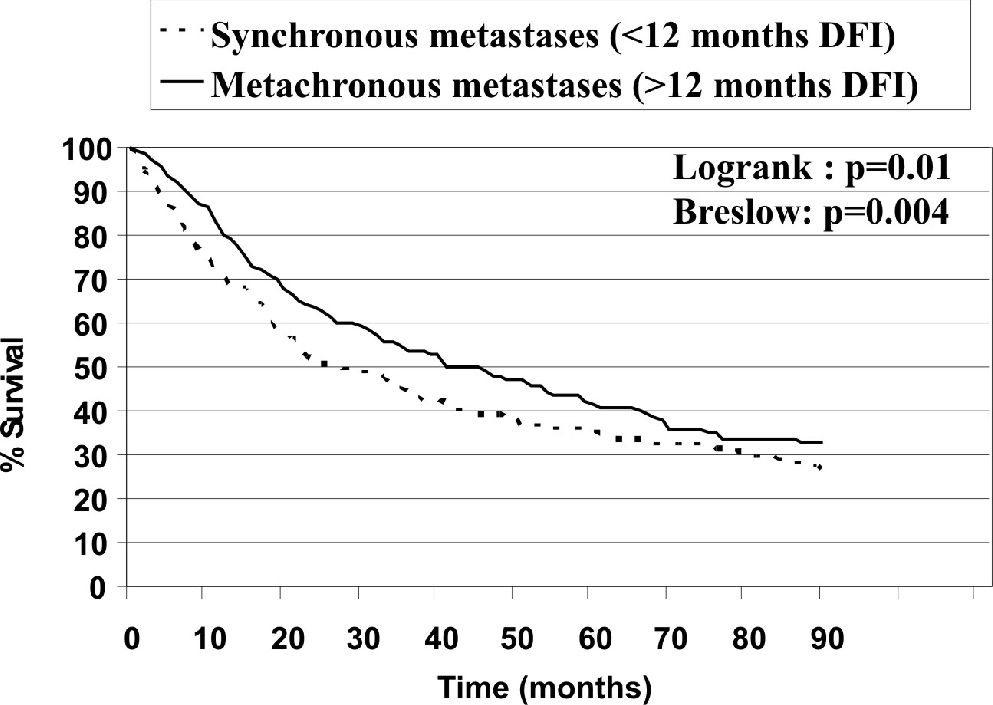
Figures 2–7 show the significant prognostic factors for overall survival, as identified by univariate analyses.
All other factors evaluated in the univariate analyses, including type of resection, response to preoperative chemotherapy, number of resected nodules, and the total number of thoracotomies in an individual patient, were not statistically significant (p>0.05).
Multivariate analysis identified the number of pulmonary nodules (p<0.001), the number of malignant nodules resected (p=0.001), and complete resection (p<0.001) as the independent prognostic factors for overall survival (Table 2).
Multivariate analysis for overall survival
| Variables included in the model | p |
|---|---|
| Primary tumor site | 0.091 |
| Histology of the primary tumor | 0.086 |
| Disease-free interval (months) | 0.063 |
| Number of nodules identified on the CT scan | <0.001 |
| Laterality of the nodules | 0.131 |
| Number of resected nodules | 0.069 |
| Number of malignant nodules resected | 0.001 |
| Diameter of greater nodule | 0.319 |
| Complete resection | <0.001 |
| Adjuvant chemotherapy after lung resection | 0.772 |
Thirty to 40% of all extrathoracic cancers lead to secondary pulmonary lesions during the course of the disease, and approximately 20% of these cases feature metastases that are confined to the lungs.2,7,12–14 Improved survival following resection of lung metastases has broadened the surgical indications for these lesions. It is likely that surgery itself impacts the outcome of patients who are candidates for a complete resection of nodules that are confined to the lungs. Therefore, resectability should be evaluated preoperatively with great care. The recent introduction of positron emission tomography using 18F- fluorodeoxyglucose (FDG PET-CT) has proven to be of clinical value for the proper staging of cancer patients. The use of this technology during the preoperative evaluation of candidates for lung metastasectomy has been shown to eliminate 21% of patients otherwise deemed resectable.15 Unfortunately, our study population did not routinely undergo this imaging evaluation because the method was not available in our institution for most patients.
The 529 patients included in this study underwent 776 thoracotomies for the treatment of lung metastases. The overall survival of patients at 90 months in our study was 30.4%, with average follow-up of 40 months. Eight patients (1.5%) were lost to follow-up.
The histological type of a primary malignant tumor has been shown to influence the results of metastasectomy in previous studies.1 In this study, survival rates were significantly better for patients with adenocarcinoma. Multivariate analysis, however, did not identify this variable as an independent prognostic factor. Better systemic therapy for patients with metastatic colorectal adenocarcinoma might explain the apparently favorable long-term outcome. Our study failed to provide sufficient evidence to support this observation.
On the basis of our univariate analysis, patients with synchronous metastases (DFI<12 months) exhibited significantly lower survival rates, compared to patients with metachronous disease, than reported in other studies.2,7 On the other hand, this factor was not significant in our multivariate analysis.
Several reports have demonstrated the prognostic importance of the number of nodules.7 The sensitivity of preoperative imaging examinations of pulmonary metastases varies depending on the technique used. Dellai et al16 evaluated the precision of the diagnosis and the exact quantification of lung nodules using computerized tomography. All of the nodules were subsequently resected, revealing a diagnostic accuracy of 51%. In a prospective study involving 182 patients in Brazil, the CT scan identified the exact number of nodules resected in 47% of all cases. However, the CT scan underestimated the actual number of lung nodules found during surgery in 28% of cases and overestimated the number in 24%.2
A recently published study by Cerfolio et al 17 showed that in 37% of patients who were candidates for video-assisted thoracic surgery (VATS), intraoperative palpation during a formal thoracotomy detected more nodules than had been identified using preoperative imaging (CT scans). VATS has a definitive role in the management of solitary pulmonary lesions that are suspected to be metastatic, but the use of thoracoscopic techniques for pulmonary metastasectomy is controversial because small metastatic foci may be missed without thorough finger palpation of the lung. Prospective studies are currently evaluating this issue in several centers.
In our study, the number of nodules exerted a significant impact on long-term overall survival using both univariate and multivariate analyses. Similar observations have been previously reported in other publications7,18
In most studies published to date, there has been no significant difference in survival of patients with metastases confined to one lung compared to bilateral lesions.19–22 However, univariate analysis in this study suggested a significant difference in survival rates between patients presenting with unilateral or bilateral disease. However, this factor was found not to be significant under multivariate analysis.
Preoperative or postoperative systemic therapy had no significant impact on long-term survival rate in this patient population, consistent with data published elsewhere.3,7,8,23–26
Complete resection was the most significant prognostic factor associated with long-term overall survival, both in univariate and multivariate analyses, as has been consistently reported elsewhere.27–35 The 90-month overall survival rate for patients who underwent complete resection in the context of metastatic disease was 35.1%, compared to 10.2% in the group who underwent incomplete resection.
Type of surgical resection and the total number of thoracotomies failed to influence the outcome of the patients, provided that complete resection was accomplished. Thus, we conclude that repeated resection may be beneficial in patients with relapses that are confined to the lung.36 As reported elsewhere, limited resection with lung parenchyma preservation is adequate to achieve clear margins and local control of the disease.8 Recently, alternative options like radiofrequency ablation have been evaluated for the management of lung metastases. Although promising, such methods are still considered experimental, since they are indicated only in limited numbers of patients, and they are usually considered clinically inappropriate for patients who can undergo a conventional resection.37
CONCLUSIONSThis study identified a group of patients who may benefit from pulmonary metastasectomy. Irrespective of other factors, all patients who present with fewer than four lung nodules should be considered candidates for surgical resection, after selection using the currently established inclusion criteria. Other patients should be individually evaluated in order to assess the impact of surgical resection on survival and long-term outcomes. Additional studies are needed to determine the role of adjuvant systemic therapy in patients stratified according to the histology of their primary tumors.