Sorafenib is an oral multikinase inhibitor that has been proven effective as a single-agent therapy in hepatocellular carcinoma, and there is a strong rationale for investigating its use in combination with other agents. Vitamin K2 is nearly non-toxic to humans and has been shown to inhibit the growth of hepatocellular carcinoma. In this study, we evaluated the effects of a combination of sorafenib and vitamin K2 on the growth of hepatocellular carcinoma cells.
METHODS:Flow cytometry, 3-(4,5-dimethyl-2-thiazolyl-2,5-diphenyl-2H-tetrazolium bromide) and nude mouse xenograft assays were used to examine the effects of sorafenib and vitamin K2 on the growth of hepatocellular carcinoma cells. Western blotting was used to elucidate the possible mechanisms underlying these effects.
RESULTS:Assays for 3-(4,5-dimethyl-2-thiazolyl-2,5-diphenyl-2H-tetrazolium bromide) revealed a strong synergistic growth-inhibitory effect between sorafenib and vitamin K2. Flow cytometry showed an increase in cell cycle arrest and apoptosis after treatment with a combination of these two drugs at low concentrations. Sorafenib-mediated inhibition of extracellular signal-regulated kinase phosphorylation was promoted by vitamin K2, and downregulation of Mcl-1, which is required for sorafenib-induced apoptosis, was observed after combined treatment. Vitamin K2 also attenuated the downregulation of p21 expression induced by sorafenib, which may represent the mechanism by which vitamin K2 promotes the inhibitory effects of sorafenib on cell proliferation. Moreover, the combination of sorafenib and vitamin K2 significantly inhibited the growth of hepatocellular carcinoma xenografts in nude mice.
CONCLUSIONS:Our results determined that combined treatment with sorafenib and vitamin K2 can work synergistically to inhibit the growth of hepatocellular carcinoma cells. This finding raises the possibility that this combined treatment strategy might be promising as a new therapy against hepatocellular carcinoma, especially for patients with poor liver tolerance.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and is the third leading cause of cancer-related mortality. The incidence of HCC is high in Asia and in parts of Africa; in developed Western countries, its incidence is low but is increasing (1-3). Unlike other tumors, HCC is often diagnosed at late stages; thus, few possibilities of curative radical treatment can be offered (4). For HCC patients, medical treatments, including chemotherapy, chemoembolization, ablation, and proton beam therapy, remain disappointing. Most patients experience disease recurrence and exhibit a 5-year relative survival rate of less than 10% (5). The prognosis for HCC patients after curative resection is better, but their 5-year survival rate is only 30 to 50% (5-7). Clearly, there is an urgent need for new therapies for this aggressive cancer.
Sorafenib (Nexavar, Bayer HealthCare Pharmaceuticals–Onyx Pharmaceuticals) is an oral multikinase inhibitor that suppresses tumor growth by acting on tumor cells and cells of the tumor vasculature (i.e., vascular endothelial cells and pericytes) (8,9). Based on its proven efficacy and tolerability in a Phase III randomized control trial, sorafenib has been approved for HCC worldwide. However, the results of clinical studies have indicated that treatment with sorafenib alone provides only minimal survival benefits for patients with advanced HCC (10). Therefore, the development of more efficacious combination therapies involving sorafenib and other agents appears to be an attractive approach for providing improved clinical outcomes in the treatment of HCC. Indeed, previous studies have shown that combination therapy with sorafenib and certain other drugs can result in synergistic or additive inhibitory effects on the growth of HCC cells.
Recent studies have revealed that vitamin K2 (VK2) has growth-inhibitory effects in a variety of human cancer cells, including HCC (11-13). In fact, a few small-sized, controlled clinical trials have reported that oral administration of VK2 reduced the development and recurrence rates of HCC and resulted in an improvement in the overall survival of patients (14,15). However, more recently, a larger-scale, randomized, controlled trial involving 548 patients did not confirm the efficacy of VK2 in suppressing HCC recurrence (16), indicating that VK2 alone may not be sufficient to produce significant clinical effects.
VK2 has been widely used for osteoporosis, and its long-term safety has been confirmed. Thus, it would be an ideal adjuvant agent for cancer therapy. Although the precise mechanism of how VK2 exerts its growth-inhibitory effects on cancer cells has not yet been determined, several reports have indicated that combined treatments with VK2 and certain other drugs, including some cytotoxic drugs, exert synergistic anti-cancer effects (11),. In this study, we report that VK2 synergistically enhances the growth inhibition effects of sorafenib on HCC cells when these two agents are administered in combination. In addition, we provide data regarding the potential mechanisms underlying these effects. These findings provide a foundation for possible future clinical evaluations of the efficacy of this combined treatment approach.
MATERIALS AND METHODSEthics statementThe experimental protocol was approved by the Institutional Animal Care and Use Committee at Southwest Hospital and was in accordance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell linesHCC cell lines (HepG2, Hep3B and HuH7) were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China) and were maintained in Dulbecco's Modified Eagle Medium (DMEM) medium supplemented with 10% fetal calf serum. Cells were cultured in an incubator with humidified air containing 5% CO2 at 37°C.
ReagentsSorafenib was supplied by the Bayer Corporation (West Haven, CT, USA). Sorafenib was dissolved in 100% dimethyl sulfoxide (DMSO) and was then diluted with DMEM to the desired concentration; the final DMSO concentration for in vitro studies was 0.1%. VK2 was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA) and was dissolved in 99.9% ethanol at a stock concentration of 50 mM. To generate working solutions, VK2 was then diluted to the appropriate concentrations with medium. Reagents were diluted with DMEM to the desired concentrations; the final DMSO or ethanol concentration for in vitro studies was 0.1%. DMSO and ethanol were added to cultures at 0.1% (V/V) as a solvent control.
MTT assayCells were seeded onto 96-well plates at a density of 1×104 cells per well. After 24 h in culture, cells were exposed to sorafenib and VK2 either alone or in combination at the indicated concentrations. Control cells were cultured in DMEM containing the corresponding concentrations of DMSO and ethanol. After 2-5 days in culture, the number of viable cells in each well was determined daily using 3-(4,5-dimethyl-2-thiazolyl-2,5-diphenyl-2H-tetrazolium bromide) (MTT) assays (Sigma-Aldrich Chemical) according to the manufacturer’s instructions.
Cell cycle analysisCells (5×105 cells/ml) were cultured in conditioning medium containing either sorafenib or VK2 alone or both in combination at the given concentrations in 6-well plates. Three days later, cells were trypsinized and harvested by centrifugation. Flow cytometric analyses of the cell cycle were performed by staining permeabilized cells with propidium iodide (PI) for DNA content.
Apoptotic analysisAnnexin V-FITC kits were used to measure the amount of apoptosis induced by sorafenib or VK2. After treatment for 36 h, cells cultured in 6-well plates were harvested, washed with PBS at 4°C and then resuspended in 100 μl binding buffer containing 5 μl Annexin V-FITC and 10 μl of PI. After a 15 min incubation at room temperature while protected from light, stained cells were analyzed by flow cytometry.
Western blot and immunoprecipitation analysisAfter 48 h in culture with sorafenib and VK2 either alone or in combination, cells were washed twice with ice-cold PBS, lysed and sonicated in RIPA buffer. The supernatant of the homogenate was used for protein determination using a BCA Protein Assay Kit (Pierce, IL, USA) and for electrophoresis. Samples with equal amounts of total protein were resolved using 10% or 12% SDS-PAGE, transferred to nitrocellulose polyvinylidene difluoride membranes (Amersham Pharmacia, Piscataway, NJ, USA), and probed with primary antibodies. The antibodies used included ERK, p-ERK, Mcl-1, cleaved caspase-3, p21, cyclin D1, and β-actin (Santa Cruz Biotechnology Inc., CA, USA). Detection and signal visualization were performed using the appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and enhanced chemiluminescence reagents (Thermo Scientific, IL, USA).
For immunoprecipitations, 1 mg of each whole-cell lysate was immunodepleted with antibody for 2 h. To this antibody complex, either protein A/G agarose or protein L agarose (Invitrogen Inc., CA, USA) beads were added for another hour and incubated at 4°C on an end-to-end shaker. The beads were washed three times with lysis buffer without protease inhibitors. Finally, 2X Laemmli buffer was added to the beads, and the samples were boiled prior to SDS-PAGE analysis.
In vivo growth inhibition assaysEGFP-expressing HepG2 cells (3×107 cells) were injected subcutaneously in two separate sites on the dorsal flanks of nude mice. Treatments were initiated when tumors reached an average volume of approximately 0.1 cm3, which was approximately seven days after cells had been inoculated. Mice were randomly divided into four groups (n = 5 animals per group): control (DMSO and ethanol, 100 μl each), sorafenib (1.25 mg/kg body weight), VK2 (2 mg/kg body weight) (19,20) and combined sorafenib plus VK2 treatment. The drugs were injected intraperitoneally once daily for 18 consecutive days. Serial changes in tumor volume were estimated every five days after the initiation of treatment. Tumor volumes were calculated according to the following formula: tumor volume = (length×width2)/2. Two days after the last injection, the animals were anesthetized and imaged using the CRi Maestro imaging system (CRi Corporation, Woburn, MA, USA).
Statistical analysisData are expressed as the mean±SD. Differences were compared using one-way ANOVAs followed by LSD t-tests. All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). p-values<0.05 were considered to be statistically significant.
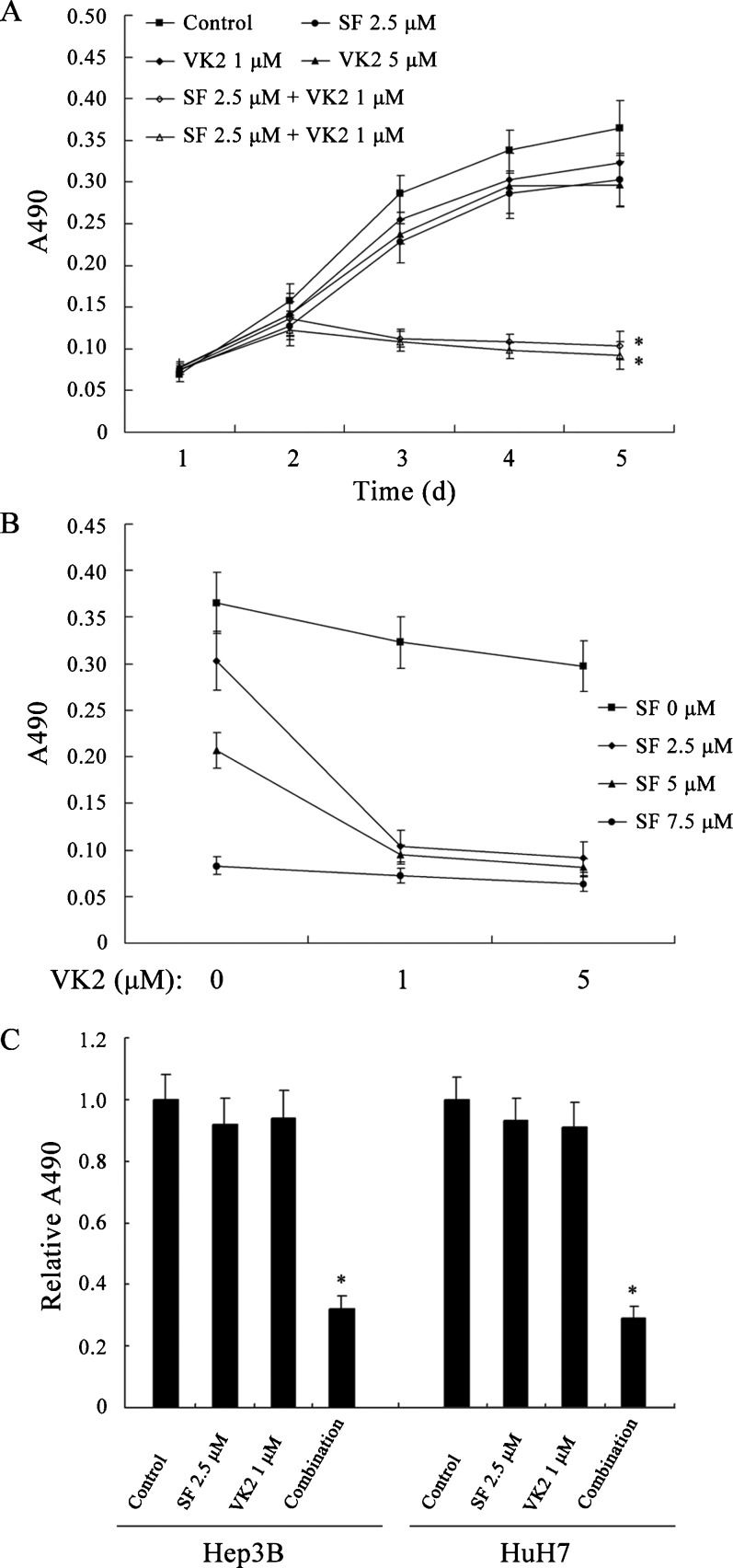
RESULTSSorafenib and VK2 synergistically inhibit the growth of HCC cellsTo assess the growth-inhibitory effects of combining sorafenib with VK2, HepG2 human HCC cells, which have frequently been used in evaluations of the effects of sorafenib, were treated with the study agents, either individually or in combination, and were then examined using MTT assays. At the concentrations tested, neither sorafenib nor VK2 alone caused significant growth inhibition compared to vehicle-treated controls (p>0.05, Figure 1A), but the combination of these two agents significantly inhibited cell growth compared to either single-agent treatment (p<0.05, Figure 1A). Several sorafenib concentrations were tested, and the growth inhibitory activity of each was enhanced by the addition of VK2 (p<0.05, Figure 1B). Similar results were observed using the Hep3B and HuH7 human HCC cell lines (Figure 1C).
Combined treatment with sorafenib and VK2 inhibits HCC cell growth. (A) Growth inhibition of HepG2 cells by sorafenib, VK2 or the two in combination. (B) The growth-inhibitory effects of VK2 in addition to various sorafenib concentrations. (C) Growth inhibition of Hep3B and HuH7 cells by sorafenib, VK2 or the two in combination. SF: sorafenib. Each point represents the mean±SD of three independent experiments. ∗ p<0.05 vs. control or single-agent treatment.
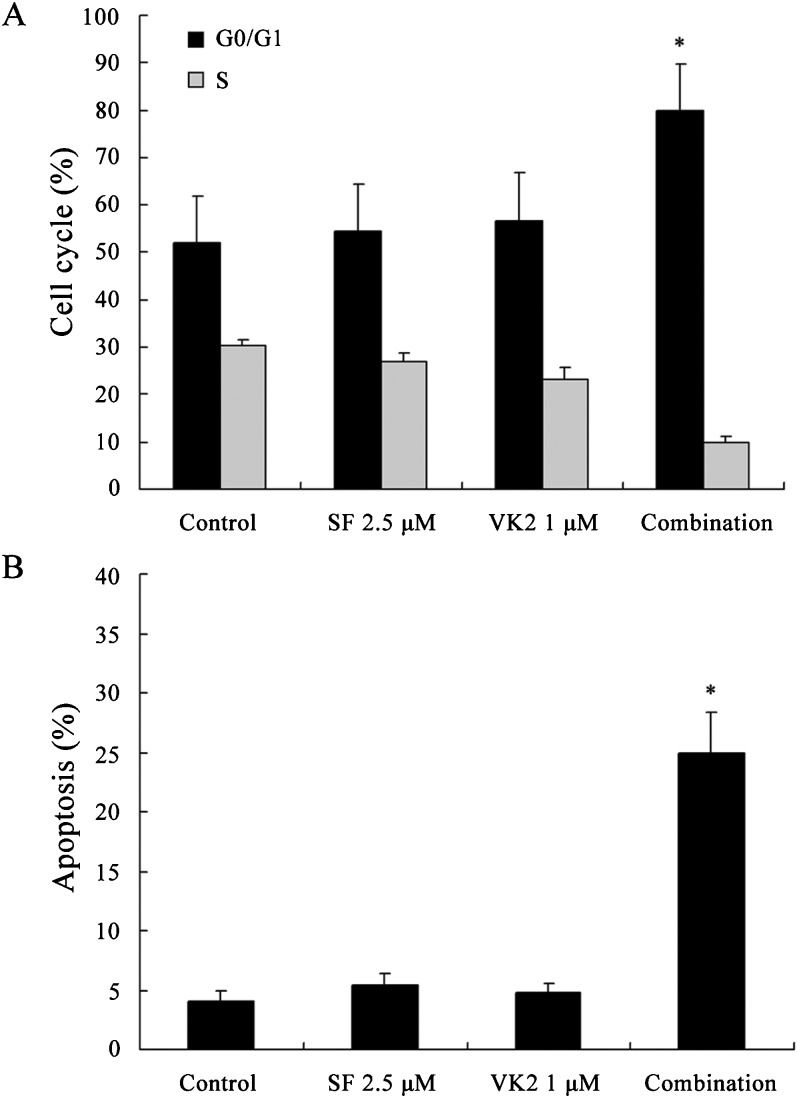
Because the combination of sorafenib and VK2 caused significant reductions in cell growth, the mechanisms underlying this effect were investigated. First, the effects of this treatment combination on cell cycle progression were examined using flow cytometry. The G1/S ratio was used as an index of G1 arrest. As shown in Figure 2A, combination treatment of 2.5 μM sorafenib and 1 μM VK2 induced a significant accumulation of cells in the G0/G1 phase when compared to control or single-agent treatments (p<0.05). To evaluate the induction of apoptosis by this combination, cells were treated with sorafenib and VK2 individually or in combination and then examined by Annexin V/PI staining followed by flow cytometry. As shown in Figure 2B, at the concentrations tested, neither sorafenib nor VK2 elicited significant apoptosis as a single agent, but the combination induced apoptosis in 24.9% of the cells (p<0.05).
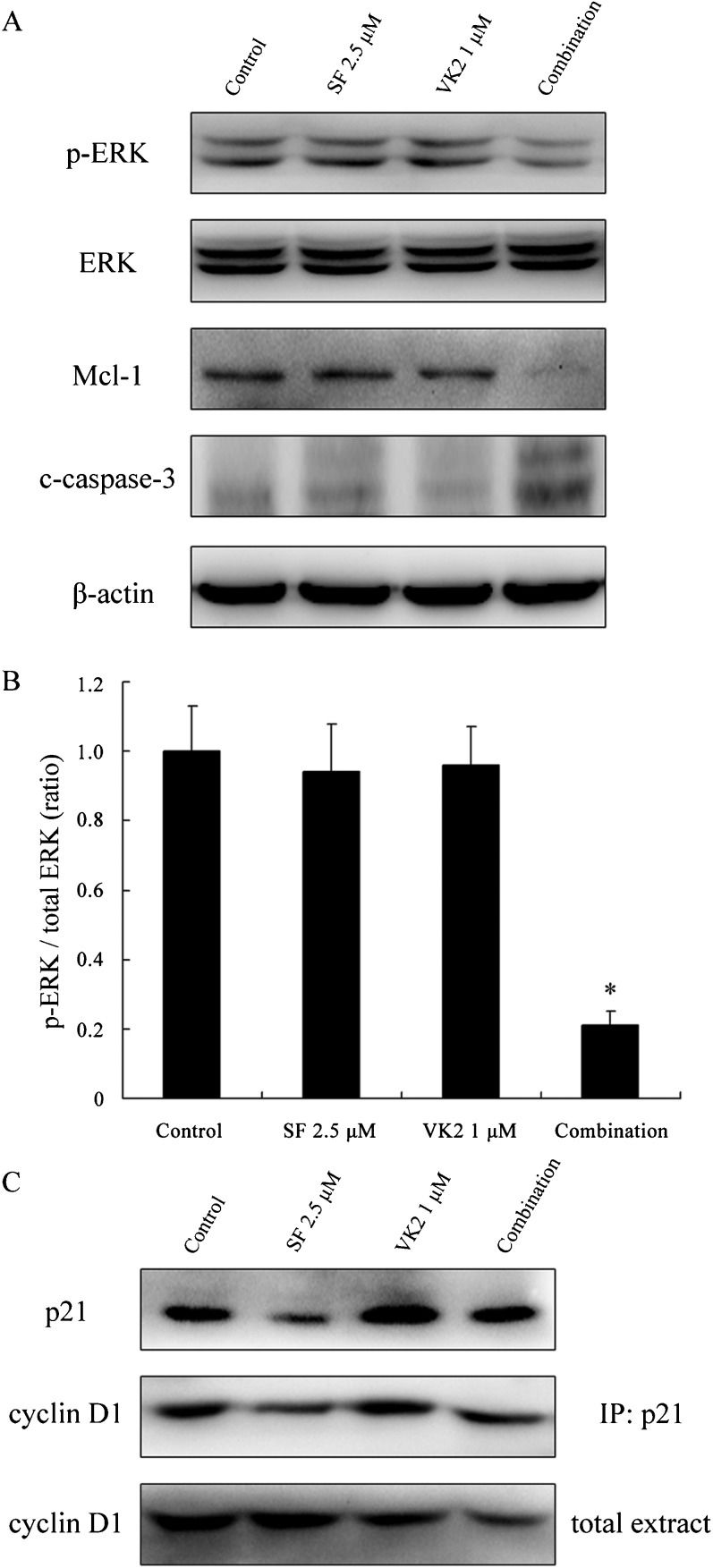
VK2 promotes sorafenib-mediated inhibition of ERK phosphorylation and caspase activationSorafenib induces growth inhibition and apoptosis in human HCC cells by inhibiting the RAF/MEK/ERK signaling pathway. In this study, the activity of the RAF/MEK/ERK signaling pathway was determined by measuring the levels of ERK phosphorylation by Western blot analysis of HepG2 cells treated with sorafenib and VK2, either alone or in combination. Neither sorafenib nor VK2 alone resulted in a significant inhibition of ERK phosphorylation at the low concentrations used, but combinations of these two agents at these concentrations caused significant reductions in phosphorylated ERK levels, but total ERK levels were unchanged (p<0.05, Figure 3A and 3B). To further examine the processes of cell death induced by combined treatment with sorafenib and VK2, we assessed expression of Mcl-1 and the marker of apoptosis, cleaved caspase-3. As shown in Figure 3A, combined treatment with sorafenib and VK2 significantly decreased the expression of Mcl-1 and resulted in remarkable caspase-3 cleavage. In contrast, no obvious decrease of Mcl-1 expression or increase in caspase-3 cleavage could be detected in samples treated with sorafenib or VK2 alone.
Western blot analysis of HepG2 cells after combined treatment with sorafenib and VK2. (A) Sorafenib and VK2 synergistically inhibited the phosphorylation of ERK, and subsequently led to Mcl-1 downregulation and caspase-3 cleavage. p-ERK: phosphorylated ERK. c-caspase-3: cleaved caspase-3. (B) The levels of p-ERK and ERK were quantitated by densitometry, and the ratios of these two proteins are displayed. Values represent the mean±SD (n = 3). ∗p<0.05 vs. control or single-agent treatment. (C) Changes in p21 expression and the levels of cyclin D1 binding to p21. Representative results from three independent experiments with similar results are shown.
The p21 protein inhibits the activity of each member of the cyclin/Cdk family, which is necessary for the transition from G1 to S phase, and thus functions as a potent inhibitor of cell cycle progression in both normal and cancer cells (21). Previous studies have revealed that sorafenib markedly decreases p21 levels in several human carcinoma cell lines, including HepG2 and HuH7, and the p21 inhibitory effect of sorafenib is independent of the RAF/MEK/ERK signaling pathway (22-25). Given the previous work indicating that VK2 suppresses the proliferation of HCC (HepG2) cells by blocking cell cycle progression from G1 to S phase via the transcriptional induction of p21 (12,26), we sought to determine if VK2 could attenuate the downregulation of p21 expression induced by sorafenib, which would be predicted to prevent the p21-mediated inhibitory effects on cell cycle progression. As previously reported, exposure to sorafenib significantly reduced the expression of p21 in HepG2 cells. However, when VK2 was combined with sorafenib, the reduction of p21 was largely abrogated. Consistent with this, VK2 increased the amount of cyclin D1 associated with p21 in HepG2 cells (as determined by immunoprecipitation), even when the total expression of cyclin D1 was reduced upon combined treatment (Figure 3C).
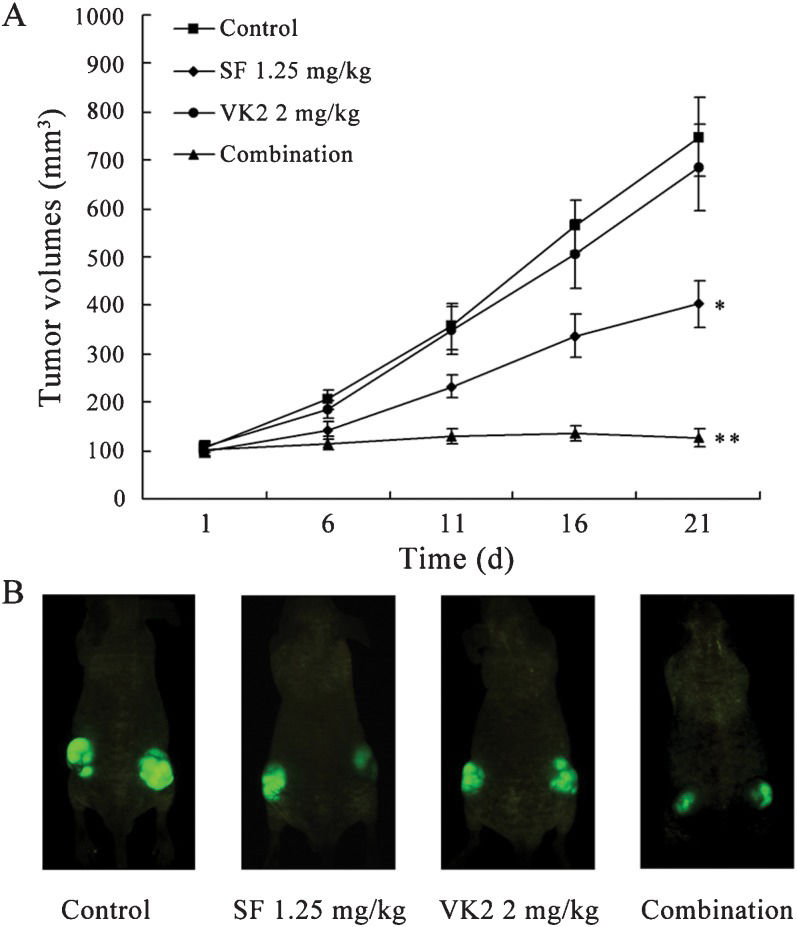
The combination of sorafenib and VK2 effectively inhibits HCC growth in vivoTo clarify whether combined treatment with sorafenib and VK2 effectively inhibits the growth of HCC cells in vivo, we next examined the effects of this combination in subcutaneous mouse models using HepG2 cells. As shown in Figure 4, sorafenib alone at the given dosage could suppress in vivo tumor growth (tumor size, p<0.05), whereas VK2 alone inhibited tumor growth only slightly. However, the combination of sorafenib and VK2 significantly inhibited tumor growth (p<0.05, vs. sorafenib alone), and some tumors were even shown to shrink significantly.
Inhibition of tumor growth in vivo by the combination of sorafenib and VK2. (A) Tumor growth curve from nude mice transplanted with HepG2 cells and treated with sorafenib, VK2 or the two in combination. Each data point represents the mean tumor volume (in mm3)±SD of five mice (10 sites) per treatment. ∗ p<0.05 vs. control; ∗∗ p<0.05 vs. treatment with sorafenib alone. (B) A representative mouse from each group using CRi imaging is shown.
Because HCC typically arises as a result of a liver damaged by cirrhosis, the livers of HCC patients frequently do not tolerate conventional chemotherapy well. Therefore, the development of selective and effective novel therapeutic strategies that are non-toxic or have low toxicity is required for HCC patients. Sorafenib is a multikinase inhibitor that targets several serine/threonine and receptor tyrosine kinases (8,9). The positive results of clinical trials have now established sorafenib as the first-ever standard systemic therapy for advanced, unresectable HCC. However, mono-therapy with this agent occasionally offers only limited survival benefits. Moreover, given that patients with cirrhotic livers exhibit poor tolerance to this drug, a large percentage of patients end up having to decrease their dose or stop taking the drug entirely. Consequently, a search for novel approaches for using sorafenib in the treatment of HCC, especially the establishment of effective combination therapies, is warranted. Vitamin K, including VK2, is theoretically an ideal combination drug for anticancer therapy. In fact, Wei et al. (19) reported that vitamin K, including VK2, can enhance sorafenib-mediated HCC cell growth inhibition in vitro and in vivo. However, in this study, Wei et al. focused on the synergistic efficiency of sorafenib and vitamin K1 and only briefly described the effects of combined treatment with sorafenib and VK2 (19). In our study, we systematically tested the synergistic effects of sorafenib and VK2 on HCC cell growth and further investigated the possible mechanisms underlying these effects.
Previous studies have revealed that VK2 itself can inhibit the RAF/MEK/ERK signaling pathway by inhibiting RAS/RAF activation, thus causing a decrease in the levels of phosphorylated ERK in several cancer cell lines, including HCC HuH7 cells (11,13). In this study, low concentrations of either sorafenib (2.5 μM) or VK2 (1 μM) alone did not significantly affect the phosphorylation levels of ERK and did not induce growth inhibition or apoptosis in HCC HepG2 cells. However, combined treatment with low concentrations of both sorafenib and VK2 resulted in significant cell growth inhibition and apoptosis and a significant reduction in ERK phosphorylation. Therefore, there appears to be a strong synergistic effect between these two drugs in inhibiting the RAF/MEK/ERK pathway.
Sorafenib has been shown to induce apoptosis at high concentrations in several human cancer cell lines by downregulating the expression of the anti-apoptotic protein, Mcl-1 (27,28). Here, we showed that single treatments with sorafenib or VK2 (at the low concentrations reported here) do not decrease Mcl-1 levels or induce apoptosis. However, when the two drugs are administered in combination, the expression levels of Mcl-1 are greatly decreased; consequently, the apoptosis marker, cleaved caspase-3, becomes detectable in HCC cells. This result indicates that when VK2 is present, even low concentrations of sorafenib can induce significant apoptosis. Thus, it is possible that patients receiving a combination of sorafenib and VK2 may achieve improved partial responses (PRs) or even stable disease (SD). The in vivo experiments in this study revealed that some nude mice bearing HCC xenografts can achieve significant tumor shrinkage after combined treatment with sorafenib and VK2, further strengthening this conclusion.
p21 is the founding member of the Cip/Kip family of cyclin-dependent kinase (Cdk) inhibitors. It plays a critical role in controlling cell cycle progression through its inhibition of cyclin/Cdk complexes, which are necessary for the transition from G1 to S phase (21,29,30). Previous studies have revealed that sorafenib induces cell cycle arrest in the G1 phase in cancer cells (31,32) through a complicated mechanism. However, it has been shown that the expression of p21 is reduced in almost all cancer cells after sorafenib exposure (22-25). Due to the critical barrier role of p21 in cell cycle progression, it is very likely that attenuating the sorafenib-mediated downregulation of p21 expression will promote the growth-inhibitory effects of sorafenib. Liu W et al. (12) has reported that VK2 activates the p21 promoter and elicits an increase in p21 expression. In this study, we demonstrate that VK2 partially prevents the down-regulation of p21 induced by sorafenib in HCC cells. Cyclin D1 is a checkpoint for the G1/S transition (33). We also observed that the levels of cyclin D1 expression are obviously reduced in HepG2 cells treated with a combination of sorafenib and VK2. Nonetheless, immunoprecipitation analysis revealed that the levels of cyclin D1 binding to p21 are greater following combination treatments than after sorafenib treatment alone, thus indicating that the rescued p21 expression exerts its growth inhibition activity by directly binding to cyclin/Cdk complexes (i.e., cyclin D/Cdk4, cyclin E/Cdk2 and cyclin A/Cdk2) during the G1/S phase progression.
In conclusion, our results indicate that sorafenib and VK2 can work synergistically to inhibit the growth of HCC cells. This finding has significant potential for clinical implications, as it suggests that combination therapy might have potential for a clinical application that could enable the use of lower and less toxic doses of sorafenib to achieve a given level of efficacy, thus improving tolerability and reducing adverse effects.
This work was supported by the National Foundation of Natural Sciences of China (No. 81101533), the China Postdoctoral Science Foundation (No. 20100481468 and No. 201104755) and the Natural Science Foundation of Hubei Province of China (No. 2011CDB017).
No potential conflict of interest was reported.
Zhang Y performed experiments, participated in the experimental design, and drafted the manuscript. Zhang B, Zhang A, Zhao Y, Zhao J and Liu J performed part of the experiments. Zhang B performed statistical analyses and assisted in drafting the manuscript. Gao J, Fang D and Rao Z participated in the experimental design and assisted in drafting the manuscript.