The aim of this study was to determine whether and how the diameter of the vein that gives rise to the inflowing vein of the esophageal and gastric fundic varices secondary to posthepatitic cirrhosis, as measured with multidetector-row computed tomography, could predict the varices and their patterns.
METHODS:A total of 106 patients with posthepatitic cirrhosis underwent multidetector-row computed tomography. Patients with and without esophageal and gastric fundic varices were enrolled in Group 1 and Group 2, respectively. Group 1 was composed of Subgroup A, consisting of patients with varices, and Subgroup B consisted of patients with varices in combination with portal vein-inferior vena cava shunts. The diameters of the originating veins of veins entering the varices were reviewed and statistically analyzed.
RESULTS:The originating veins were the portal vein in 8% (6/75) of patients, the splenic vein in 65.3% (49/75) of patients, and both the portal and splenic veins in 26.7% (20/75) of patients. The splenic vein diameter in Group 1 was larger than that in Group 2, whereas no differences in portal vein diameters were found between groups. In Group 1, the splenic vein diameter in Subgroup A was larger than that in Subgroup B. A cut-off splenic vein diameter of 8.5 mm achieved a sensitivity of 83.3% and specificity of 58.1% for predicting the varices. For discrimination of the varices in combination with and without portal vein-inferior vena cava shunts, a cut-off diameter of 9.5 mm achieved a sensitivity of 66.7% and specificity of 60.0%.
CONCLUSION:The diameter of the splenic vein can be used to predict esophageal and gastric fundic varices and their patterns.
Posthepatitic cirrhosis is common worldwide and results in portal hypertension (PHT), due to an increase in intrahepatic resistance combined with an increase in portal and hepatic arterial blood flow. To decompress the portal venous system, portosystemic collateral vessels are formed in PHT (1–4). The collaterals are mainly composed of esophageal and gastric fundic varices, which contribute to massive hemorrhage of the upper alimentary tract (5,6). Thus, it is crucial to evaluate the originating veins of inflowing veins of the collateral circulation for the determination of appropriate treatments.
The originating vein is typically either the portal or splenic vein. Doppler ultrasound can not only detect the portal vein (PV) but also measure its diameter, flow direction, and flow velocity (7). However, this procedure has not been widely used in clinical settings, due to its lack of reproducibility and poor accuracy resulting from intra- and interobserver variation (8,9). Magnetic resonance imaging is probably as accurate as angiography, but several of the rarest pathways (e.g., pleuropericardial or thoracic wall varices) may be missed at the time of MR imaging (10,11).
The development of multidetector-row computed tomography (MDCT) has resulted in an improved spatial resolution and the elimination of motion artifacts due to its ability to acquire images rapidly and continuously during a single held breath (12,13). The capacity for the postprocessing of imaging data with a variety of three-dimensional (3D) reformatting techniques (e.g., maximum intensity projection (MIP), multiplanar reformation (MPR), and volume rendering (VR)) can facilitate the identification of the originating veins and the distribution of portosystemic collateral vessels in patients with liver cirrhosis; therefore, MDCT is probably the optimal imaging technique in this setting (14–16). Almost all of the reported studies have sought to illustrate the anatomical distributions of portosystemic collaterals. To our knowledge, there have been no reports focusing on how to predict esophageal and gastric fundic varices and their patterns with the diameters of the originating vein of the inflowing vessels, as measured with MDCT. Therefore, the aim of this study was to determine how to use the diameter of the originating vein to predict the varices and their patterns to develop a better understanding of and to prevent massive hemorrhage of the upper alimentary tract.
MATERIALS AND METHODSEthics statementThis study was approved by the institutional ethics review board of our university hospital, and written informed consent was obtained from all participants prior to initiation of the study.
Patient populationPatients were enrolled in this study according to the following inclusion criteria: (1) PHT secondary to posthepatitic cirrhosis resulting from hepatitis B, as confirmed by clinical data and laboratory examinations and imaging studies performed according to the American Association for the Study of Liver Diseases (AASLD) practice guidelines on chronic hepatitis B (2007) (17); (2) lack of prior treatment for esophageal and gastric fundic varices caused by the absence of upper gastrointestinal bleeding; (3) absence of portal vein emboli, hepatic artery-portal vein fistula, and hepatic carcinoma; and (4) available thoracicoabdominal triple-phase enhanced CT scans.
Between January 2010 and July 2011, 106 consecutive patients (74 men and 32 women; mean age, 53.5 years; age range, 16 - 78 years) who met the inclusion criteria and agreed to take part in the study were recruited. The common clinical manifestations included a feeble state, abdominal distension, dyspepsia and dull pain in the liver. According to the Child-Pugh classification, the cohort was composed of 45 patients classified as Child-Pugh A, 35 as Child-Pugh B, and 26 as Child-Pugh C.
The cohort was divided into two groups based on whether they had esophageal and gastric fundic varices, as confirmed by enhanced MDCT. The group with varices was subdivided into two subgroups according to whether the varices were or were not associated with portal vein-inferior vena cava (PV-IVC) shunts. Patients with esophageal and gastric fundic varices served as Group 1 (n = 75), and patients without collaterals served as Group 2 (n = 31). In Group 1, patients with isolated esophageal and gastric fundic varices served as Subgroup A (n = 30), and patients with varices in combination with PV-IVC shunts served as Subgroup B (n = 45).
Computed tomography techniqueParticipants in our study underwent thoracicoabdominal triphasic enhancement scans with a 16-row MDCT (Aquilion, Toshiba Medical Systems, Tokyo, Japan). Prior to CT image acquisition, a 21-gauge plastic cannula (B. Braun Melsungen AG, Melsungen, Germany) was placed into an antecubital vein, and 400-600 ml of water was immediately used as negative oral gastric contrast material. A breath-hold thoracoabdominal plain scan was obtained. Subsequently, a 1.5-ml/kg bolus of iopamidol (Ultravist 300, Iopamidol, Schering, Germany) was injected with an automated pump injector (MEORAD-Stellant, MEORAD Company, Pittsburg, Germany) at a rate of 3.0 ml/s through the 21-gauge cannula into the antecubital vein. Triphasic enhancement CT scans were subsequently commenced 25, 45, and 65 s after the start of the injection. The first enhanced acquisitions were used to acquire hepatic arterial phase images, and the third acquisitions were used to acquire portal venous phase images. The following parameters were used for the second and third sets of enhanced images: peak voltage of 120 kVp, tube current of 120-380 mA, collimation of 7 mm, pitch of 1.3, matrix of 512×512 mm, and a reconstructed section thickness of 1 mm. The second or third sets of enhanced images were obtained during suspended respiration for 10-15 s, and the thoracoabdominal scanning coverage along the z-axis ranged from 60-75 cm. The parameters used for non-enhanced images and the first set of enhanced images were similar to those used for the second and third sets of images with the exception of the 5-mm reconstructed section thickness.
Image data analysisThe data derived from the third enhanced acquisition were transferred to an image processing workstation (Aquilion Multislice CT, Toshiba, Tokyo, Japan) for reconstruction. The display parameters, including width, level, opacity, and brightness, were chosen subjectively to visualize these portosystemic collaterals most effectively. For MPR, a slab of 7-10 mm was applied to avoid the interference of the vertebral bodies. The images were reviewed by two radiologists working in consensus, including an experienced radiology professor (the corresponding author, who has 13 years of experience in thoracoabdominal radiology) and an experienced radiologist (the first author, who has six years of experience in radiology), with emphasis on the patterns of esophageal and gastric fundic varices, the inflowing vessels and their originating veins. The patterns of the varices were evaluated in some cases with PV-IVC. The inflowing vessels were the left gastric vein and the posterior and short gastric veins. Because there was a degree of difficulty in differentiating the posterior gastric and short gastric veins, we regarded these as the posterior/short gastric vein. The originating veins were the PV and splenic vein (SV).
The PV and SV diameters were measured on axial CT images using the liver window setting (window width, 250 HU; window level, 70 HU). PV diameter was measured at its midpoint as determined on MPR images, and the diameter of the SV was measured at a point 1 cm from the confluence of the superior mesenteric vein and SV (18). Furthermore, the diameters were measured repeatedly on the 1st and 30th days after the scan by the abovementioned radiologists working in consensus to test intraobserver concordance. To minimize operator-dependent bias, each set of imaging data was analyzed with the observers having no knowledge of the patients' clinical data.
Statistical analysisStatistical analysis was performed using the Statistical Package for Social Sciences 13.0 (SPSS Inc., Chicago, IL, USA). A p-value less than 0.05 was considered to be statistically significant. All of the measurement results are given as the means ± standard deviations. The precision of PV and SV measurements was tested by the concordance correlation coefficient (rc). rc values of more than 0.85, between 0.50 and 0.85, and less than 0.50 indicated very good concordance, moderate concordance, and poor concordance, respectively. An independent-samples t test was used to evaluate the differences in the diameters of the originating veins between groups or subgroups. If there were significant positive findings, the cut-off value was then determined using receiver operator characteristic (ROC) analysis to predict the esophageal and gastric fundic varices and their patterns.
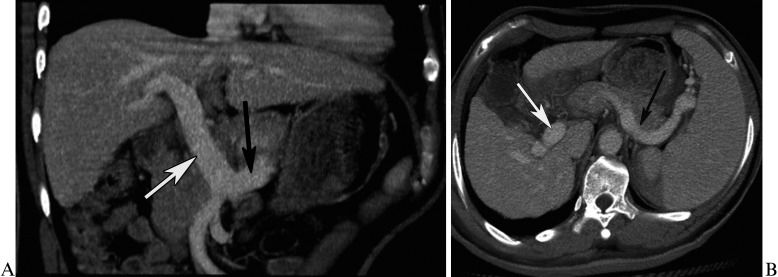
RESULTSEsophageal and gastric fundic varicesIn Group 1, 15 patients had esophageal varices, 12 patients had gastric fundic varices, and 48 patients had both esophageal and gastric fundic varices. The primary inflowing vessel was the left gastric vein, which originated from the PV or SV (Figure 1A and B), and the posterior/short gastric vein, which originated from the SV (Figure 2A and B). The details of the inflowing vessels and the originating veins are provided in Table 1. The originating vein was the PV in 8% of patients (6/75), the SV in 65.3% (49/75), and both the PV and SV in 26.7% (20/75). In Group 2, all patients lacked collaterals and exhibited ectasia of the PV and SV (Figure 3A and B).
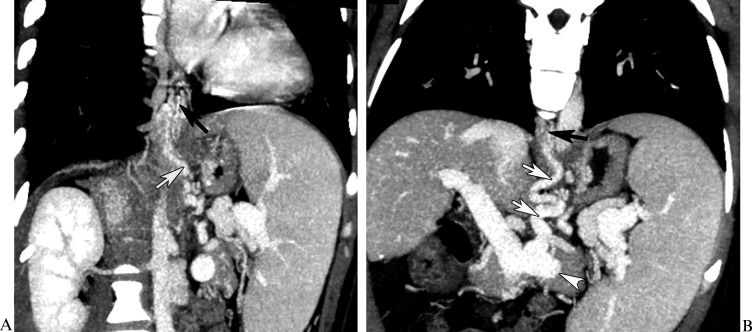
In a 56-year-old female with esophageal and gastric fundic varices secondary to posthepatitic cirrhosis, the computed tomography multiplanar reformation reconstruction images demonstrated esophageal varices (A and B, black arrow), and the inflowing vessel is the left gastric vein (A and B, white arrow) originating from the splenic vein (B, white arrowhead).
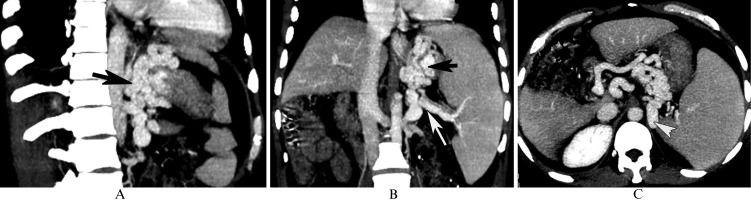
In a 48-year-old male with gastric fundic varices secondary to posthepatitic cirrhosis, the computed tomography multiplanar reformation reconstruction images show gastric fundic varices (A and B, black arrow) originating from the splenic vein (B, white arrow), which are associated with nephrogastric shunts (C, white arrowhead).
The inflowing veins and their originating veins in esophageal and gastric fundic varices in Group 1 (n = 75).
| Shunts | Inflowing vein | Originating vein | |
|---|---|---|---|
| PV (n) | SV (n) | ||
| Esophageal varices | LGV (n = 15) | 6 | 9 |
| Gastric fundic varices | P/SGV (n = 12) | 0 | 12 |
| Esophageal and gastric fundic varices | LGV and P/SGV (n = 48) | 20* | 48* |
Note: LGV = left gastric vein; P/SGV = posterior/short gastric vein; PV = portal vein; SV = splenic vein. * Both PV and SV were origination vessels in 20 patients with esophageal and gastric fundic varices.
In Group 1, all patients in Subgroup A had esophageal and gastric fundic varices without PV-IVC shunts. Subgroup B comprised 11 patients with nephrogastric shunts (Figure 2C), 11 with splenonephric shunts, 6 with venae parumbilicales varices, 4 with paravertebral varices, and 13 with two or more shunts.
Intraobserver concordance of the originating vein diameter measurementsIn this cohort, the mean SV diameters were 9.42 ± 2.75 mm (range, 3.61 - 18.40) for the first measurements and 9.40 ± 2.73 mm (range, 3.58 - 18.13) for the repeated measurements with an rc value equal to 0.97. The mean PV diameters were 14.13 ± 2.68 mm (range, 9.21 - 25.08) for the first measurements and 14.28 ± 2.85 mm (range, 9.00 - 25.00) for the repeated measurements with an rc value equal to 0.90. Therefore, the intraobserver concordance of the diameter measurements was sufficient, and the first measurements were used as the final diameter values.
Comparison of PV and SV diameters between groups or subgroupsThe mean SV diameters were 9.76 ± 2.95 mm (range, 3.61 - 18.40) in Group 1 and 8.52 ± 1.88 mm (range, 5.00 - 13.25) in Group 2, and the mean SV diameter in Group 1 was larger than that in Group 2 (p = 0.03). In Group 1, the mean SV diameters were 10.60 ± 2.28 mm (range, 7.11 - 14.53) in Subgroup A and 9.20 ± 3.22 mm (range, 3.61 - 18.40) in Subgroup B. The SV diameter in Subgroup A was larger than that in Subgroup B (p = 0.04).
However, there were no significant differences in PV diameters between Group 1 and Group 2 (14.32 ± 3.46 mm versus 13.74 ± 1.59 mm, p = 0.38), and no differences in PV diameters were found between Subgroups A and B (14.87 ± 2.34 mm versus 13.96 ± 4.02 mm, p = 0.27).
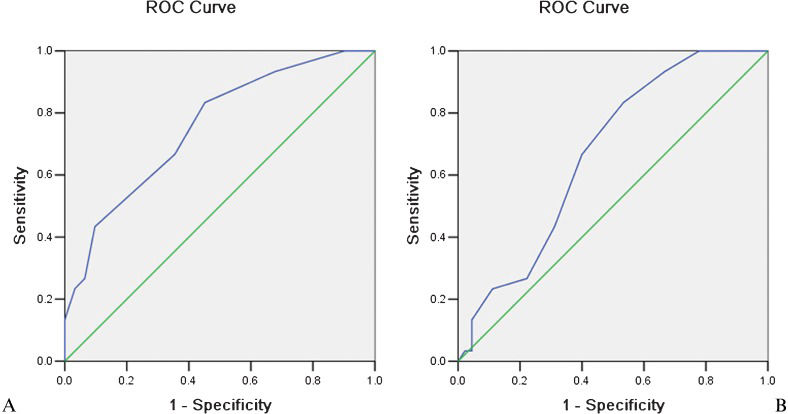
The SV diameter cut-off values used to predict esophageal and gastric fundic varices and discrimination of their patternsIn Group 1, 63 of 75 patients (84.0%) with esophageal and gastric fundic varices had an SV diameter ≥8.50 mm, whereas 13 of 31 patients (41.9%) in Group 2 with no collaterals had an SV diameter ≥8.50 mm. For determining whether esophageal and gastric fundic varices have occurred, the cut-off SV diameter of 8.5 mm achieved a sensitivity of 83.3%, specificity of 58.1%, and AUC of 0.75 (Figure 4A).
Receiver-operating characteristic (ROC) curves demonstrate the use of a cut-off splenic vein diameter of 8.5 mm in predicting the presence of esophageal and gastric fundic varices (A). A threshold diameter of 9.5 mm was used to discriminate isolated esophageal and gastric fundic varices from the varices associated with portal vein-inferior vena cava shunts (B).
In Group 1, 20 of 30 patients (66.7%) with isolated esophageal and gastric fundic varices had an SV diameter ≥9.50 mm compared with 18 of 45 patients (40.0%) with varices associated with PV-IVC shunts. For discrimination of the isolated esophageal and gastric fundic varices from the varices associated with PV-IVC shunts, the cut-off value of 9.5 mm achieved a sensitivity of 66.7%, specificity of 60.0%, and AUC of 0.67 (Figure 4B).
DISCUSSIONEsophageal and gastric fundic varices, which can contribute to massive hemorrhage of the upper alimentary tract, are the most common collateral vessels in PHT patients (5,6). An increasing number of treatments, such as endoscopy and intravascular interventional techniques, require radiographic examination of the varices. After endoscopic variceal ligation, mucosal gastric fundic and esophageal varices diminished markedly, but collateral veins around the esophagus and gastro- and/or spleno-renal shunts remained unchanged (19). Thus, visualization of the originating veins of the inflowing vessels is crucial to guide further treatments (5,6).
Compared with other modalities used to evaluate varices, MDCT portography has proven to be the optimal imaging technique, due to its high spatial resolution, rapid image acquisition, and powerful postprocessing of the imaging data (14–16). In this study, the patterns and originating veins of the inflowing vessels of gastric fundic and esophageal varices were depicted accurately on CT-MPR images, which further demonstrates the advantages of MDCT portography for evaluating varices.
The primary inflowing vessels of esophageal and gastric fundic varices found in our study were the left gastric vein and posterior/short gastric vein. The predominant originating vein of the inflowing vessels was the SV, rather than the PV. Furthermore, we found that the diameter of the SV was associated with the pattern of the varices, which is not consistent with published studies (20–22). Bolognesi et al. (20) and Yin et al. (21) demonstrated that the diameters of the PV and SV were the key criteria for the diagnosis of PHT and that there was a positive linear correlation between the diameters and the severity of PHT. However, Li et al. (22) reported that the diameters of the PV and SV were not sensitive enough to be used as markers of PHT severity.
In cirrhotic patients, due to portal outflow obstruction (i.e., elevated intrahepatic portal vascular resistance) and increasing pressure of the portal venous system, the diameters of the PV and SV may initially enlarge. When the diameters of the PV and SV dilate to a peak point with a concomitant increase of the portal venous system pressure, the common collaterals (esophageal and gastric fundic varices) send blood flow from their originating veins to the collaterals, which, in turn, results in a decrease in diameter (2–4). Therefore, the diameter of the SV (the primary originating vein) may be associated with the presence of varices, and the SV diameters in patients with esophageal and gastric fundic varices may be larger than in those without varices. In addition, we can presume that the presence of collaterals other than the esophageal and gastric fundic varices further decreases the diameters of the originating veins. Thus, the SV diameters in patients with varices associated with PV-IVC shunts may be smaller than in those without shunts.
Because of the significant difference in SV diameter between patients with and without esophageal and gastric fundic varices, the SV diameter measurements can be used as criteria to predict the presence of varices. Sensitivity and specificity values of more than 58% were achieved with a cut-off SV diameter of 8.5 mm for differentiating PHT with and without esophageal and gastric fundic varices based on the present data and using ROC analysis. Moreover, the SV diameter measurements could also be used as criteria with which to differentiate isolated esophageal and gastric fundic varices from the varices associated with PV-IVC shunts. A cut-off value of 9.5 mm yielded sensitivity and specificity values of approximately 60%.
However, this study had certain limitations. Portography was performed using a 16-slice CT scanner, and the time resolution and density resolution of this scanner should be improved. Nonetheless, these factors did not impact our assessment of the collaterals or the measurement of PV and SV diameters (13,15).
In conclusion, we used portal venography with MDCT to visualize the originating vein of vessels entering esophageal and gastric fundic varices secondary to posthepatitis cirrhosis. On CT portovenography, the SV rather than PV diameter was associated with the presence of varices. The SV diameter could be used as the criterion with which to predict the varices and to identify the varices associated with PV-IVC shunts.
As depicted by multidetector-row computed tomography, we found that the splenic vein rather than the portal vein may be the primary vein entering esophageal and gastric fundic varices secondary to posthepatitic cirrhosis. A splenic vein diameter cut-off value (8.5 mm) could be helpful in identifying varices. Furthermore, the diameter of the splenic vein could be helpful in discriminating the varices associated with portal vein-inferior vena cava shunts; a good cut-off diameter might be 9.5 mm.
AUTHOR CONTRIBUTIONSZhou HY, Chen TW and Zhang XM contributed to the conception and design of the study; to the generation, collection, assembly, analysis and interpretation of the data; to the drafting and revision of the manuscript; and approved the final version of the manuscript. Wang LY, Zhou L and Zeng NL contributed to generation, collection, assembly, analysis and interpretation of the data, and approved the final version of the manuscript. Dong GL, Li H, Chen XL and Li R contributed to the generation, collection, assembly, analysis and interpretation of the data, to the drafting and revision of the manuscript; and approved the final version of the manuscript.
This study was supported by the National Natural Science Foundation of China (Grant No. 81050033), Key Projects in the Sichuan Province Science and Technology Pillar Program (Grant No. 2011SZ0237), and the Science Foundation for Distinguished Young Scholars of Sichuan Province, China (Grant No. 2010JQ0039).
No potential conflict of interest was reported.