The aim of our study was to investigate the impact of typical and atypical antipsychotic drugs on leptin concentration in blood and changes in the receptor expression in the hypothalamus of male Wistar rats.
METHODS:From the age of 13 to 18 weeks, three groups of 20 animals were fed an average dose of 3.5 ± 0.03 mg/ kg body weight (BW) haloperidol; 30.6 ± 0.22 mg/kg BW clozapine; or 14.9 ± 0.13 mg/kg BW ziprasidone in ground food pellets containing 15% fat. Twenty control animals received no drugs. Blood samples were taken at week 14, 16, and 19. Locomotor activity and exploratory behavior were measured using the alcove test at weeks 15 and 17. The expression of the hypothalamic leptin receptor in rat brains was determined by using a Western blot.
RESULTS:Rats medicated with haloperidol and ziprasidone showed a significantly decreased percentage weight gain and food consumption. We observed no differences in the alcove test, but locomotor activity was significantly reduced in the haloperidol group. Except for rats in the clozapine and ziprasidone groups, after 2 weeks of drug application, we found no changes in the leptin blood concentrations among the four groups or animals within each group. Moreover, we did not find specific differences in hypothalamic leptin receptor expression among the groups.
CONCLUSION:We concluded that in male Wistar rats during this treatment period, the tested drugs did not act directly on the leptin regulatory system. We recommend further studies using long-term treatment of different rat strains.
Antipsychotic drug medication is an important therapeutic option for the treatment of patients suffering from schizophrenia and other psychoses. Based on their mechanism of action, antipsychotics are classified as either typical or atypical drugs. Medication with typical antipsychotic drugs (haloperidol is applied most frequently) can be accompanied by severe extrapyramidal side effects. Atypical antipsychotic drugs like clozapine usually cause no extrapyramidal side effects but can lead to unwanted weight gain.1,2 Interestingly, ziprasidone, a newer atypical antipsychotic drug, is not linked with weight gain.3 As increased body weight may be associated with an increase in blood leptin concentration,4 researchers proposed that an impaired leptin secretion or signalling might play a role in antipsychotic-induced weight gain.
Adiposity and weight gain are closely associated with severe health problems, including hypertension, type II diabetes, and coronary disease. Moreover, weight gain under antipsychotic treatment may reduce compliance and cause patients to abandon the medication. This endangers treatment success and causes a risk of critical deterioration of the psychiatric condition. Therefore, the availability of antipsychotic drugs without the side effect of weight increase appears to be fundamental for effective and cost-efficient treatment of schizophrenia. The mechanisms underlying antipsychotic-induced weight gain, however, are poorly understood. Plausible explanations include reduced physical activity, metabolic changes resulting in reduced calorie burn-off, stronger appetite resulting in increased food intake,5,6 and differences in receptor binding profiles.7
In the complex physiological network regulating food intake, energy expenditure, and fat storage, researchers have found that leptin plays a key role. Leptin, a polypeptide product of the obese (OB) gene, is secreted by adipocytes. Its expression and secretion shows a strong positive correlation with body fat mass and adipocyte number and size.8 Leptin, together with other hormones, including glucocorticoids, amylin, and insulin, plays an important role in regulating food intake, energy expenditure, body weight homeostasis,9 reproduction function, and neuroendocrine responses.10 The lack of leptin results in hormonal and metabolic alterations and a dramatic increase in body weight.11 One of the primary tasks of leptin is sending information to the central nervous system – especially hypothalamic areas – about the amount of energy stored in the adipose tissue. In the brain, leptin interacts with the Ob-R receptor found in both rodents and humans. The leptin receptor is a class I cytokine receptor, a single-membrane-spanning protein that has several isoforms due to alternative splicing.12 The short form Ob-Ra and the long form Ob-Rb are both abundantly expressed in the hypothalamus.
Haloperidol, which produces little or no weight gain, acts primarily on the dopamine D2 receptors, whereas clozapine has a high affinity with the histamine H1 and the serotonin 5-HT2C receptors. Both H1 and 5-HT2C receptors are implicated in the control of food intake and body weight.13 Furthermore, the histamine H3 receptor seems to play a key role in the regulation of food intake and hunger by inhibiting other neurotransmitters, such as serotonin, noradrenaline, and acetylcholine, and by controlling the synthesis and release of histamine.14 Studies have shown increased leptin concentrations in psychiatric patients treated with clozapine or conventional antipsychotics15 and also in noncompliant patients, but not in antipsychotic-naïve, first-episode schizophrenic patients.16 Haupt et al.17 detected no differences in the plasma leptin concentrations of patients medicated with antipsychotic drugs and healthy controls. However, Thakore et al.18 showed that drug-naive and drug-free patients with schizophrenia exhibit increased intra-abdominal fat and that people with schizophrenia appear to be overweight more often than those in the general population.19
Experimental studies on rodents can help researchers to understand the mechanisms underlying antipsychotic-induced metabolic changes. Numerous studies of both classical and atypical antipsychotic drugs have demonstrated changes in body weight, food consumption, or body fat composition associated with the release of leptin. Increasing serum leptin levels in male Sprague Dawley rats after long-term treatment using haloperidol and olanzapine added to food were substantiated in a study by Minet-Ringuet et al.20 Chronic clozapine application in female Wistar rats showed a negative correlation between serum leptin levels and the given doses (i.p.).21 In our study, we aimed to clarify the effect of typical and atypical antipsychotic drugs on blood leptin secretion and hypothalamic leptin receptor expression. These results were correlated with weight gain and food and water consumption after a 6-week period of selective treatment with haloperidol, clozapine, or ziprasidone mixed with food.
METHODSAnimals and housing conditionsAll experiments were carried out in accordance with the local laws governing animal experimentation and approved by Bezirksregierung Düsseldorf authorities. On postnatal day 21 (PD 21), male pups of Wistar rats (outbreed strain, animal facility Düsseldorf, Heinrich-Heine-University of Düsseldorf) were removed from their mothers and were individually housed on PD 85 with free access to water and dry, ground food pellets containing 15% fat. They were maintained on a 12∶12 light/dark cycle (lights off at 20.00 h) at a temperature of 21°C and 60% humidity. To limit the possible impact of individual weight gain of the test animals, the rats derived from litters with identical pup numbers, so each group was equally composed of rats with different parentage.
Preliminary dose testBased on the results of Kapur et al.,22 which showed that the current dosing of haloperidol in animal studies is inappropriately low in chronic dosing studies, we tested 3 different daily doses of each antipsychotic drug added to ground food pellets: 2, 5, and 8 mg haloperidol (Haloneural, Hexal, Germany)/ kg body weight (BW); 20, 40, and 50 mg clozapine (Leponex, Novartis, Germany)/ kg BW; and 10, 20, and 25 mg ziprasidone (Zeldox, Pfizer, Germany)/ kg BW. Each dose was tested in 3 animals beginning at the age of 12 weeks. Weight gain and food consumption in g per 100 g BW were measured during a 2-week period, and the serum level of haloperidol, clozapine, and N-desmethylclozapine were determined by HPLC in ng/ml.
Assessment of food and water consumptionStarting at week 11 (PD 71), the animals were weighed twice per week (a = first weighing on Tuesday and b = second weighing on Friday), and their weekly water consumption was calculated. Each animal's food consumption was measured each day and averaged for a week, including the loss of ground pellets in the litter. Based on the preliminary dose studies starting at week 13 (PD 85), each of the 20 animals in a trial group received an average daily dose of 3.5 ± 0.03 mg/kg BW haloperidol, 30.6 ± 0.22 mg/kg BW clozapine, or 14.9 ± 0.13 mg/kg ziprasidone in a measured quantity of ground pellets. Serum levels of haloperidol and clozapine by HPLC showed that this route of administration leads to pharmacologically active plasma levels. The control group (20 animals) was fed only ground pellets.
Assessment of serum leptin levelsOn PD 96 (week 14) and PD 110 (week 16), blood samples were taken from the retro-orbital plexus of anesthetised animals with 150 mm glass Pasteur pipettes (Brand, Germany).
The blood samples were centrifuged, and the leptin blood serum level was determined by using a rat leptin assay kit (IBL, Japan) in ng/ml. On PD 127 (week 19), the animals were sacrificed by CO2 inhalation, and their blood was sampled by cardiac puncture. The brains were removed and immediately frozen in 2-methylbutane (Merck, Germany), cooled down by liquid nitrogen, and stored at -80°C. The serum levels of leptin in the heart blood were determined using a rat leptin assay kit (IBL, Japan) in ng/ml. Additionally, the serum levels of haloperidol and clozapine (clozapine and N-desmethylclozapine) in the heart blood were analyzed using HPLC in ng/ml. Ziprasidone was not measurable since an HPLC method has not been established.
Horizontal locomotor activity and alcove testAround week 11 and 12, the animals were habituated to the experimental setup. Locomotor activity under antipsychotic drug medication was measured compared to that of non-treated controls using an open field activity test in a standard box (40 × 40 × 30 cm). Animal movement was restricted to the square acrylic inner cage that was closed with a perforated lid. On PD 102 (week 15) and PD 116 (week 17), respectively, the animals were individually placed into the center of the field and observed for 20 min in the dark. Locomotion was assessed automatically using photocells. In the alcove test, which is a refined open field exploratory test,23 the rats were individually placed into a small dark start box connected to a brightly-lit open field by a guillotine door. The door of the box was opened and the latency to leave the box was measured. Each trial ended after 2 min and was repeated after 30 min. The scores of the two tests were totalled.
Semiquantitative detection of the leptin receptorHypothalami of the brains, which were frozen and stored at -80°C, were dissected, weighed, and homogenized in 0.25 µl ice-cold lysis buffer24 (50 mM Hepes pH 7,9; 10% glycerol; 1 mM EDTA; 1 MM sodium pyrophosphate; 1 mM sodium fluoride; 1 mM sodium vanadate; 1 mM PMSF; containing a proteinase inhibitor, diluted 1∶50, Sigma P 8340). Nonidet P-40 and Triton X-100 were added to a final concentration of 1%. After 30 min on ice, the homogenates were centrifuged at 4°C at 13000 Ü for 10 min. Proteins in the supernatant were precipitated with A/G Plus-Agarose (Santa Cruz Biotechnology, sc-2003) following the manufacturer's specifications. The precipitates were centrifuged after 10 min on ice at 13000 Ü for 10 min at 4°C. The supernatant was discarded and the sediment resolved in an SDS buffer (0.005 DTT and 0.01 DTE resolved in 50 µl distilled water and 450 µl 25% SDS diluted 1∶6 in 0.05 M Tris-HCl pH 8.8). The proteins were stored at -80°C. The protein content of each sample was determined. For a Western blot analysis, 30 µg protein were resolved by SDS-PAGE (7.5% polyacrylamide in the separating gel) and transferred to a PVDF membrane (Invitrolon PVDF 0.45 µm pore size, Invitrogen, Carlsbad USA). Membranes were incubated in TBS-T with 7.5% nonfat milk overnight at 4°C followed by 2 h at room temperature with mouse monoclonal OB-R antibody (diluted 1∶400, Ob-R B-3; Santa Cruz Biotechnology), which detects the short and long isoforms of the receptor, and goat polyclonal actin antibody (1∶800, Akt 1 C-20; Santa Cruz Biotechnology). To identify immunoreactive bands, membranes were subsequently incubated with HRP-conjugated secondary antibodies (goat anti-mouse HRP IgG and donkey anti-goat HRP IgG, respectively; diluted 1∶5000 for detection of Ob-R and 1∶10000 for detection of actin 1, Santa Cruz Biotechnology). Signals were visualized using a chemiluminescence detection kit (Santa Cruz Biotechnology) on Kodak BioMax Light film (Kodak Industries, Chalon-sur-Saone, France). Resultant autoradiographs were quantified by densitometry using Gene Snap and Gene tools software (Syngene, Synoptics Ltd, United Kingdom).
Statistical analysisAll data are presented as mean ± SEM. The data were analysed by two-way analyses of variance (ANOVA) with GROUP as the between-group factor and WEEKS as the repeated measures factor unless otherwise specified. Posthoc tests with α-adjustment for repeated measurement were carried out for single measurement points.
A bivariate correlation procedure using the Spearman-Rho coefficient was carried out to test the relationship between food consumption and the level of leptin in the serum. We also tested the correlation between the level of leptin in the heart blood and the animal's weight before death and the correlation between the level of leptin and the amount of haloperidol and clozapine (clozapine and its main metabolite N-desmethylclozapine) in the serum.
RESULTSPreliminary dose testWe found an inverse dose / body weight gain correlation of haloperidol and ziprasidone over 2 weeks with equal results for food consumption. Animals fed with clozapine showed the highest percentage of weight gain at 40 mg/ kg BW with equal results for food consumption. But a one-way ANOVA revealed no differences among the groups for body weight gain and food consumption (all p ≥ 0.05).
At daily 2 mg/kg BW, haloperidol was not detectable in the serum using HPLC. At 8 mg/kg BW, the side effects of haloperidol, e.g., apathy, were very pronounced. Therefore, we chose a daily dose of 5 mg/kg BW haloperidol. At 20 mg/kg BW, we detected only a low serum level of clozapine, and its metabolite was not discernible. After discovering a lower weight gain and food intake at 20 mg and 50 mg clozapine, we decided to apply a daily dose of 40 mg/kg BW. We chose a daily dose of 20 mg/ kg BW of ziprasidone (corresponding to a real weekly dose of 14.6 mg/kg BW), according to Pillai et al.25.
Preliminary dose-finding test.
| mg/kg BW/day | % BW | food intake/100g BW (g) | drug serum level (ng/ml) | N-desmethyl serum level (ng/ml) |
|---|---|---|---|---|
| 2 haloperidol | 20.8±1.8 | 5.5±0.3 | 0 | |
| 5 | 18.9±2.5 − | 5.3±0.4 − | 65.8±15.1 + | |
| 8 | 17.5±0.8 | 5.1±0.1 | 94.26±12.1 | |
| 20 clozapine | 27.5±0.6 | 5.7±0.1 | 55.5±9.6 | 0 |
| 40 | 28.8±1.8 | 6.3±0.4 | 75.5±3.1 + | 36.7±7.7 + |
| 50 | 26.1±0.8 | 6.0±0.2 | 110.6±28.1 | 182.0±26.1 |
| 10 ziprasidone | 20.2±0.9 | 5.4±0.2 | ||
| 20 | 18.0±1.8 − | 5.3±0.3 − | ||
| 25 | 16.0±1.2 | 5.0±0.1 |
One finding of the preliminary dose test was the fact that rats did not eat all of the allocated food each day. Therefore, in the main trial, we calculated the real weekly dose and averaged it over the test period. We calculated a mean dose of 3.5 ± 0.03 mg haloperidol/ kg BW, 30.6 ± 0.22 mg clozapine/ kg BW and 14.9 ± 0.13 mg ziprasidone/ kg BW.
Weight gainBefore starting the medication at the age of 11 and 12 weeks (data are not shown in Figure 1 because we refer to the percentage body weight gain on the first weighing after beginning the drug application), the animals' weight showed a significant difference over time, with weights increasing for animals in all groups [F(3,165) = 699.3, p < 0.00000], but there were no differences among the groups [F(3,55) = 0.41, p = 0.75] and in the WEEKS∗GROUP [F(9,165) = 0.6, p = 0.8]. This indicates that the alterations in body weight among the groups after week 12 resulted from the antipsychotic medication.
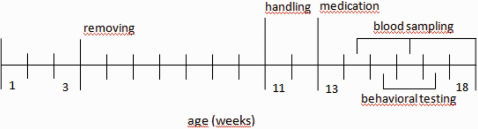
Timeframe of experimental setup. The male Wistar pups were removed from their mothers at PD 21, housed together, and received ground pellets at this time. At week 11 and 12, the rats were weighed twice per week and were habituated to the activity and alcove box. At week 13, the animals were medicated with haloperidol, clozapine, or ziprasidone (n = 20 for all groups) or were fed only ground pellets (controls, n = 19). At week 12 and 14, blood was sampled. At weeks 15 and 17, activity and anxiety behavior was tested. After 6 weeks of medication, the rats were anesthetized and their blood and brains were removed.- Timeframe of experimental setup. The male Wistar pups were removed from their mothers at PD 21, housed together, and received ground pellets at this time. At week 11 and 12, the rats were weighed twice per week and were habituated to the activity and alcove box. At week 13, the animals were medicated with haloperidol, clozapine, or ziprasidone (n = 20 for all groups) or were fed only ground pellets (controls, n = 19). At week 12 and 14, blood was sampled. At weeks 15 and 17, activity and anxiety behavior was tested. After 6 weeks of medication, the rats were anesthetized and their blood and brains were removed.
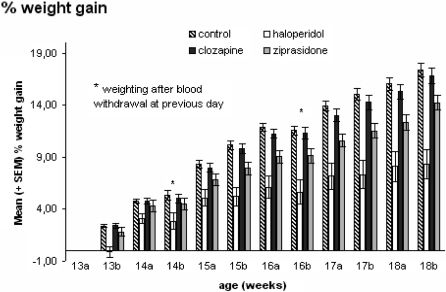
We found a continuous weight gain in all four groups over 6 weeks of drug application. The percentage increase in weight referred to the first weighing after the onset of antipsychotic medication and showed significant differences (Fig.1) for WEEKS [F(11,803) = 537.54, p < 0.00000], WEEKS∗GROUP [F(33,803) = 11.11, p < 0.00000], and GROUP [F(3,73) = 14.6, p < 0.00000], with the highest increase in the control group, followed by the clozapine and the ziprasidone groups, and the lowest gain for the haloperidol group.
Post-hoc tests revealed that the values of the haloperidol group were significantly lower compared to controls (week 13b p = 0.001, week 15a p = 0.004, week 15b p = 0.0002, week 16a p = 0.0002, week 16b p = 0.0003, week 17a p = 0.0002, week 17b p = 0.0001, week 18a p = 0.0002, and week 18b p = 0.00003, respectively) and the clozapine group over all weeks except for week 14 (week 13b p = 0.001, week 15b p = 0.001, week 16a p = 0.001, week 16b p = 0.001, week 17a p = 0.002, week 17b p = 0.001, week 18a p = 0.001, and week 18b p = 0.00008, respectively). Animals with ziprasidone medication showed decreased body weight compared to the control group from week 16 to week 18 (week 16a p = 0.003, week 17a p = 0.001, week 17b p = 0.002, and week 18a p = 0.004), but there were no differences between the ziprasidone and the clozapine groups and between animals fed with ziprasidone and haloperidol. In the tested period, no differences were detectable between the control and the clozapine group (all p>0.004).
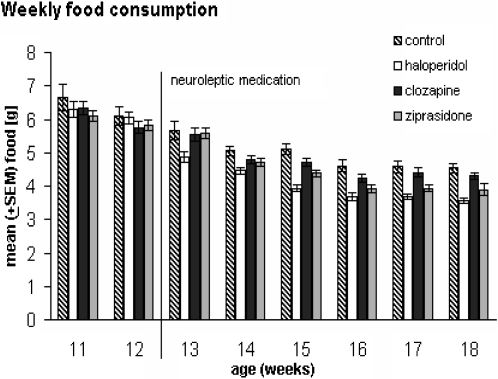
Food consumptionWe averaged the food consumption of each day over a week, based on 100 g BW (Fig.2), avoiding the influence of the individual animals' body weight on the results. Taking into consideration the loss of ground pellets in the litter, we subtracted the pellet parts, screened from the litter, from the total weekly food quantity.
Percentage weight gain of male Wistar rats: control (n = 19) and animals treated with haloperidol, clozapine, and ziprasidone (n = 20 for all groups). All animals were weighed twice per week. Depicted is the weight after application of the antipsychotic drugs from week 13 to week 18. No difference was found between the control and clozapine groups. The haloperidol-treated animals gained less weight than the control and clozapine group directly after medication, whereas the ziprasidone group gained less weight after 4 weeks of treatment than the control group with p≤ 0.004 considered significant.- Percentage weight gain of male Wistar rats: control (n = 19) and animals treated with haloperidol, clozapine, and ziprasidone (n = 20 for all groups). All animals were weighed twice per week. Depicted is the weight after application of the antipsychotic drugs from week 13 to week 18. No difference was found between the control and clozapine groups. The haloperidol-treated animals gained less weight than the control and clozapine group directly after medication, whereas the ziprasidone group gained less weight after 4 weeks of treatment than the control group with p≤ 0.004 considered significant.
Statistical analysis revealed a significant decrease in food consumption per 100 g BW over time [F(1,55) = 40.48, p < 0.00000], but there were no differences for the WEEKS∗GROUP [F(3,55) = 2.01, p = 0.12] and GROUP [F(3,55) = 0.66, p = 0.58] over the 2 weeks of testing before antipsychotic application. Therefore, we concluded that the differences among the four groups were a consequence of drug medication.
After application of antipsychotic drugs, food consumption continuously decreased over time [F(5,365) = 179.41, p < 0.00000]. For WEEKS∗GROUP [F(15,365) = 2.96, p = 0.0002] and among the groups [F(3,73) = 9.72, p = 0.00002], we also found significant differences. The highest food consumption level occurred in the control group, and the lowest was in the haloperidol group. Posthoc tests showed that at week 13 the groups did not differ with reference to food consumption (all p > 0.008). From week 14 to 18, the food intake decreased significantly for the haloperidol group compared with the control group (p = 0.004, p < 0.00000, p = 0.0003, p = 0.0002 and p = 0.00001, respectively). At week 15 and 18, we also discovered differences between the control and ziprasidone-treated animals (p = 0.001 and p = 0.008, respectively). At weeks 15, 17, and 18, the level of food consumption of the rats with haloperidol medication differed from those treated with clozapine (p = 0.0004, p = 0.004 and p = 0.001, respectively). We detected no such differences between the clozapine and ziprasidone groups, between the control and clozapine groups, and between the haloperidol and ziprasidone groups (all p > 0.008, accounting for the α-adjustment).
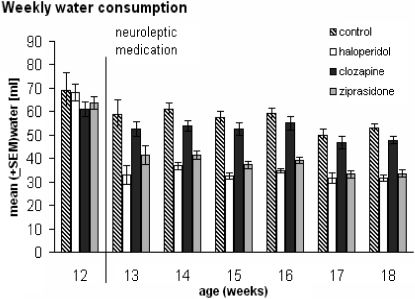
Weekly water consumptionWater consumption was measured weekly. To avoid a bias due to individual development of body weight over time, water consumption was related to 100g BW (Fig. 3).
Weekly food consumption based on 100 g BW of male Wistar rats: control (n = 19) and animals treated with haloperidol, clozapine, or ziprasidone (n = 20 for all groups). All animals showed decreased food consumption from week 11 to week 18 with no differences before medication. Animals under haloperidol medication ate less than the control and clozapine groups after 1 week of application. Ziprasidone-treated rats ate less than the controls after 3 week of medication. We found no differences between the control and clozapine groups with p≤ 0.008 considered significant.- Weekly food consumption based on 100 g BW of male Wistar rats: control (n = 19) and animals treated with haloperidol, clozapine, or ziprasidone (n = 20 for all groups). All animals showed decreased food consumption from week 11 to week 18 with no differences before medication. Animals under haloperidol medication ate less than the control and clozapine groups after 1 week of application. Ziprasidone-treated rats ate less than the controls after 3 week of medication. We found no differences between the control and clozapine groups with p≤ 0.008 considered significant.
The one-way ANOVA showed no differences among the groups at week 12 (p = 0.27). Thus, we concluded that further differences in the course of the experiment between the four groups were a consequence of antipsychotic medication.
After the administration of the drugs, an ANOVA for repeated measures showed no differences between WEEKS∗GROUP [F(15,170) = 1.26, p = 0.23]. Significant differences were found from week 13 to week 18 (WEEKS [F(5,170) = 22.74, p < 0.00000]) and among the drug groups (GROUP [F(3,34) = 47.1, p < 0.00000]. Controls and clozapine-medicated rats did not differ in drinking behavior from week 13 to week 18 (all p > 0.008). In addition, the behavior of the haloperidol-medicated animals was no different than the behavior of rats medicated with ziprasidone (all p > 0.008). Significant differences were found between controls and clozapine-medicated animals on the one side and haloperidol- and ziprasidone-treated rats on the other side during the course of the complete testing period (p ≤ 0.008 at all measuring points). Any correlation between the real body weight and the water consumption was not verifiable.
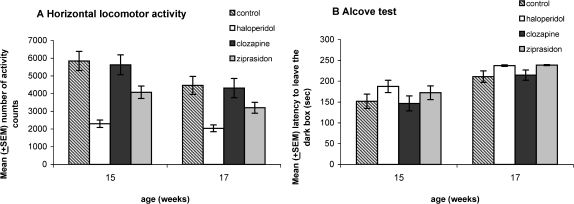
Horizontal locomotor activity and alcove testTo examine the sedating impact of the neuroleptics, we investigated locomotor activity and exploratory behavior in the alcove test at weeks 15 and 17.
We found a decrease in the activity counts (Fig. 4A) from week 15 to 17 [F(1,73) = 40.59, p < 0.00000] with high levels in the control group and the group treated with clozapine, followed by the group treated with ziprasidone, and an unexpected low activity in the haloperidol group. The activity counts for weeks 15 and 17 were significantly different among the control, clozapine, and ziprasidone groups (p = 0.005, p = 0.001 and p = 0.001, respectively), but not in the haloperidol group (p = 0.18). We also found differences for the WEEKS∗GROUP [F(3,73) = 3.01, p = 0.035] and GROUP [F(3.73) = 12.54, p < 0.00000]. The animals treated with haloperidol in week 15 (p = 0.00002 vs. control group, p = 0.00007 vs. clozapine group and p = 0.001 vs. ziprasidone group) and week 17 (p = 0.001 vs. control group, p = 0.004 vs. clozapine group and p = 0.02 vs. ziprasidone group) differed from the other groups, whereas the controls and the animals treated with clozapine and ziprasidone did not show differences.
Weekly water consumption based on 100 g BW of male Wistar rats: control (n = 19) and animals treated with haloperidol, clozapine, or ziprasidone (n = 20 for all groups). All animals showed slightly decreased water consumption from week 11 to week 18 but no differences before medication. Animals under haloperidol and ziprasidone medication drank less than the control and clozapine groups after 1 week of application. We found no differences between the control and clozapine groups with p≤ 0.008 considered significant.- Weekly water consumption based on 100 g BW of male Wistar rats: control (n = 19) and animals treated with haloperidol, clozapine, or ziprasidone (n = 20 for all groups). All animals showed slightly decreased water consumption from week 11 to week 18 but no differences before medication. Animals under haloperidol and ziprasidone medication drank less than the control and clozapine groups after 1 week of application. We found no differences between the control and clozapine groups with p≤ 0.008 considered significant.
In the alcove test, we found an increase in the latencies (Fig. 4B) spent in the dark box from week 15 to 17 as expected ([F(1,73) = 45.73, p < 0.00000]). Posthoc tests revealed that the increase was not significant for all groups (p = 0.007 for the control groups; p = 0.003 for the haloperidol group, p = 0.005 for the clozapine group, and p = 0.001 for the ziprasidone group). No differences were found for the WEEKS∗GROUP [F(3,73) = 0.21, p = 0.89] and GROUPS [F(3,73) = 3.73, p = 0.05].
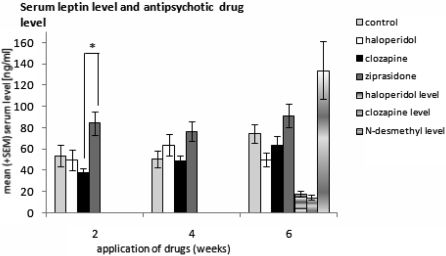
Blood leptin and drug concentration and leptin receptor expression in the hypothalamusWe found a mean value of 18.3 ± 2.7 ng/ml haloperidol, 14.4 ± 2.2 ng/ml clozapine, and 134.0 ± 26.9 ng/ml N-desmethylclozapine in the serum after 6 weeks of antipsychotic drug application.
Serum levels of leptin increased over time in the control, clozapine, and ziprasidone groups, whereas the haloperidol-medicated group showed a maximum after 4 weeks of drug application. Although the overall leptin concentration in blood did not change over time (WEEKS [F(2,134) = 2.76, p = 0.067]), differences were found for the WEEKS∗GROUP [F(6,134) = 2.18, p = 0.049] and GROUPS [F(3,67) = 5.31, p = 0.002]. Posthoc tests showed significant differences between the clozapine and ziprasidone groups two weeks after medication (p = 0.005). All other groups yielded no significant differences (p > 0.016).
To investigate the drug's impact on leptin receptor expression in the hypothalamus, we performed Western blot and immunohistochemical experiments. No differences in receptor expressions were discovered among the four groups.
Correlation between food consumption, drug quantity, weight before death and blood leptin concentrationsWe could not substantiate a significant correlation between food consumption and leptin blood concentrations. No correlation was detected between antipsychotic drug levels and leptin concentration.
The bivariate correlation procedure showed a significant positive effect (Sperman's Rho correlation coefficient 0.567, p = 0.01) between the weight of the animals (weight before death) treated with haloperidol and leptin concentration. No correlation was found in the other groups.
DISCUSSIONIn this study, medication of male Wistar rats with haloperidol, clozapine, and ziprasidone caused heterogenous effects on weight gain, food and water intake, and sedation compared to controls. The blood leptin concentration, however, was not different among the four groups, and there were no age-dependent effects within the groups. Moreover, there were no significant differences in the leptin receptor expression in the hypothalami of medicated or untreated animals.
Medication of rats with clozapine did not induce the typical side effects known from observations in humans, including weight gain,19 decreased physical activity,26 and abnormal eating behavior like food craving or binge eating.6 However, we cannot exclude the fact that clozapine induced body fat deposition in rats without increasing their body weight or metabolic abnormalities as described.20,21,27 To clarify this point, in future studies of weight gain after antipsychotic drug medication in rats, visceral white fat depositions have to be determined.
In most rodent studies, antipsychotics were administered by using daily gavage, drinking water,28 food, and intraperitoneal or subcutaneous injections. Drug administration by food is the least-invasive application manner, reduces handling stress, and allows the exact determination of food and drug intake.29 The plasma half-life of neuroleptic drugs lasts much longer in humans than in rodents. According to Minet-Ringuet et al.20 it is plausible that by mixing antipsychotics into the food, more consistent drug blood levels are maintained, so this is a more promising approach to model drug application in the human.
Weight gain, food and water consumptionThe observed effects on weight gain and food and water consumption are likely to be due to the medication itself because no differences were detectable among the groups before the application of the antipsychotic drugs. Compared to control animals, rats medicated with clozapine showed a small decrease in weight gain as well as food and water consumption although these differences were not significant. Rats with haloperidol medication gained less body weight than all other groups immediately after application. We measured significant differences in weight gain among animals in the haloperidol group compared to controls and animals medicated with clozapine but not in those medicated with ziprasidone. After one week of drug treatment, rats with haloperidol medication ate and drank significantly less than controls and the clozapine group. Hartfield et al.30 showed that low doses of haloperidol decreased drinking of a fat emulsion while clozapine was associated with an increased intake. Yoshida et al.31 observed a suppression of body weight gain in male rats under a low oral haloperidol application that was not accompanied by changes in food consumption but by reduced water intake. In contrast, Minet-Ringuet et al.32 found no differences in body weight, body composition, and food consumption among animals treated with a lower dose of haloperidol and controls. Lee et al.33 also observed small changes in food or water consumption at low doses of haloperidol and clozapine. Thus, we conclude that our observed sharp effects of haloperidol on weight gain and food and water consumption are due to the high drug dose applied (3.5 ± 0.03 mg/ kg BW). We have not observed vacuous chewing movements following the dose of haloperidol used in the present study, so it is unlikely that the decreased food and water consumption among rats under the haloperidol application was due to a malfunction of the mandible. However, Sanci et al.34 found significantly increased vacuous chewing movements in haloperidol-medicated rats (1.5 mg/kg i.p.), suggesting D1 receptor stimulation and possibly receptor supersensitivity.
After three weeks of ziprasidone medication, rats showed a significant decrease in weight gain compared to controls, but there was no statistical difference compared to the clozapine or haloperidol groups over the whole experiment period. Food consumption was significantly reduced in comparison to the controls after two weeks of drug treatment, and water consumption decreased after one week in comparison to the control and clozapine groups. Therefore, in contrast to the animals treated with haloperidol, the effects on weight gain among those in other groups were possibly caused by decreased food consumption.
As described by Davoodi et al.35 and Fell et al.,36 ziprasidone intake is not associated with weight gain and hyperphagia in either humans or rodents. Although ziprasidone is an atypical antipsychotic drug, its effects on weight gain and food and water consumption show a delay in time comparable to that seen with the use of the typical antipsychotic haloperidol. Terry et al.37 reported that in rats, ziprasidone has less pronounced behavioral effects, and neurochemical deficits appear to be more delayed.
Mechanisms underlying drinking behavior remain unclear. There is some evidence that nitric oxide plays a role in the regulation of drinking.38 Studies have shown that the brain's L-arginine/NO pathway may be involved in the central effect of leptin on feeding behavior and body weight gain in mice38 and chickens.40 These mechanisms must be substantiated by further investigations.
We found a positive correlation for all 4 groups between food intake and body weight before blood sampling. Only the animals medicated with haloperidol showed a positive correlation between body weight and serum leptin levels after 6 weeks of drug application. While our data do not exclude the possibility that a chronic application of haloperidol might have a decreasing impact on number, size, or leptin secretion rate of adipocytes proportional to the body weight, it must be noted that Minet-Ringuet et al.41 did not observe any effects of haloperidol on cell size, lipolytic activity, and glucose transport activity in rat adipocytes after oral treatment for five weeks.
Horizontal locomotor activity and alcove testIn the alcove test, which assesses fear and anxiety, we found no differences among animals in the four groups. Rats with haloperidol medication showed a highly significant decrease in locomotor activity compared to those in the other groups. Interestingly, there was a habituation effect over time in controls and in rats medicated with clozapine and ziprasidone, while the low activity counts of the rats in the haloperidol group remained unchanged over the test period. This corroborates earlier studies31,42,43 showing a reduced activity under haloperidol application closely related to the mesolimbic and mesocortical nervous system. Sorge44 also showed that clozapine had no effect on the spontaneous locomotor activity of rats.
Antagonistic blockade of dopamine D2 receptors using haloperidol led to a suppression of locomotor activity in rats by decreasing the excitability of the spinal motor centers.45 The study of Huang et al.46 found that locomotion and appetitive behavior are differently sensitive to haloperidol, which allows the suggestion that separable D2 mechanisms are involved in regulating the feeding behavior. Nitric oxide seems to play a role not only in drinking regulation but also in motor behavior, probably due to interference with dopaminergic neurotransmission.47 Decreased water consumption can cause tiredness, obnubilation, and apathy in humans. Both systems and their interaction may explain the very low activity level in male rats under haloperidol medication. The reduced activity under haloperidol was not solely induced by a dysfunction of water balance – in animals treated with ziprasidone there was reduced water consumption, but no decreased locomotor activity was detectable despite the high affinity of this drug to bind to dopamine D2 and D3 receptors.
Serum leptin level and receptor expression in the hypothalamusClozapine and olanzapine seem to cause the greatest risk of weight gain for respectively treated patients, whereas ziprasidone or haloperidol generally account for no or minimal increase in weight.48 As shown by Atmaca et al.,4 leptin is involved in the regulation of body weight, and its concentration in blood seems to be increased under clozapine medication. However, Haupt et al.17 found no differences in plasma leptin concentrations calculated separately according to gender and concluded that the increased weight under antipsychotic medication was not correlated with a malfunctioning leptin secretion or sensitivity, respectively. Kraus et al.49 measured lower leptin levels in schizophrenics compared to depressive patients. He found that the decreased leptin concentration was independent of drug medication.
Our results showing a lower serum leptin level after a 6-week treatment with haloperidol were similar to the findings of Minet-Ringuet et al.32 Except for the clozapine and ziprasidone groups at the time of the first blood sampling after 2 weeks of drug application, we found no changes in the leptin blood concentration among the four groups of animals, although animals medicated with haloperidol exhibited decreased body weight. Therefore, we conclude that the drugs have no effect on the leptin secretion of the adipocytes. We had the same results as Hauner et al.,50 finding no in vitro effect of clozapine on leptin expression and its release from the adipocytes. In line with these findings, Minet-Ringuet et al.41 reported that haloperidol had no effect on cell size, lipolytic activity, and glucose transport activity in rat adipocytes after a five-week oral treatment.
Animal models do not always mimic clinical findings but can help researchers understand underlying mechanisms. Due to metabolic differences between humans and rodents, our model naturally has its limits. One of them is the finding of the applied drugs having no effect on serum leptin levels in male Wistar rats, which seem to be less sensitive to drug-induced metabolic changes than Sprague Dawley32 or Han Wistar rats.21 Both of the named rat strains showed drug-induced differences in serum leptin levels.
Because we lacked specifications, we chose drug doses corresponding to the results of the preliminary dose test. Jennings et al.51 showed that the increased c-Fos expression in the rat brain induced by oral administration of 10 mg/ kg ziprasidone may be consistent with reported clinical effects. In human patients, the chosen dose of ziprasidone is normally 10 times higher than the haloperidol dose. However, lacking data on the antipsychotic efficacy in our rat model is a strong limitation of our study. Furthermore, our treatment period may have been too short to show significant alterations in leptin levels, and studies using long-term treatment are recommended.
Leptin plays a significant role in energy homeostasis.52 It signals the status of fat stores to the hypothalamus and interacts with orexigenic and anorexigenic central pathways. Strong receptor expression was demonstrated in regions of the hypothalamus.53 Leptin receptors expressed in hypothalamic areas process the intensity of the leptin signal and effector systems, including the sympathetic nervous system, and supervise energy intake and expenditure.
Although food and water consumption was significantly decreased in rats medicated with haloperidol and ziprasidone compared to controls, we found no changes in hypothalamic leptin receptor expression, even after prolonged antipsychotic drug medication. Liu et al.54 found that the blood concentration of leptin in obese rats was increased and that gene expression levels of Ob-Ra and Ob-Rb in the hypothalamus were significantly reduced. Leptin blood concentration in obese rats had a significant negative association with both Ob-Ra and Ob-Rb gene expression levels in the hypothalamus, and this suggests that a leptin mediated down-regulation of Ob-R expression is one of the leptin-resistant mechanisms for maintaining obesity. In summary, the results of our study suggest that the atypical antipsychotic drugs clozapine and ziprasidone do not influence the peripheral and central pathway of leptin in Wistar rats. It is plausible that these drugs have a direct effect on the rat brain, especially mediated by NPY-containing neurons of the hypothalamus.55 Oral antipsychotic treatment with these drugs did not cause weight gain in male rats. This finding is concordant with the results of Baptista et al. 55 who found no conclusive evidence that leptin was involved in inducing obesity in patients after atypical antipsychotic drug medication.
We thank Dr. A. Treiber, director of the TVA, Heinrich-Heine-Universität, Düsseldorf, and her assistants for excellent support regarding the animal experiments. Dr. M. Jänner, Rheinische Kliniken, Düsseldorf, kindly shared her expertise in the statistical analysis of our results.