Postprandial lipemia is characterized by an increased concentration of circulating lipids after fat intake and is an independent risk factor for cardiovascular disease. Exercise is known to reduce postprandial lipemia and its negative clinical outcomes.
OBJECTIVE:This study investigated the effect of intense intermittent versus moderate continuous exercise using the same energy expenditure in postprandial lipemia.
MATERIALS AND METHODS:Twenty healthy men (aged 21.5±3.5 years) performed a random sequence of either rest or 500 Kcal tests separated by a minimum 48 h interval as follows: (a) no exercise (control), (b) intense intermittent exercise, or (c) moderate continuous exercise. Each test series was completed 30 min before ingestion of a high-fat meal (1 g fat/kg). Venous blood was collected before and at 1, 2, 3 and 4 hours after the high-fat meal. Postprandial lipemia was assessed using the area under the curve approach as well as a kinetic profile of mean lipid variables. Statistical significance was tested at the p≤0.05 level.
RESULTS:With both statistical approaches, intense intermittent and moderate continuous exercises were both effective in reducing postprandial triglycerides; however, only intense intermittent exercise reduced the levels of postprandial very low density lipoprotein. Intense intermittent and continuous exercise produced lower levels of insulinemia using the area under the curve analysis only.
CONCLUSION:Intense intermittent or continuous exercise with an energy expenditure of 500 kcal completed 30 min before ingestion of high-fat meal reduced postprandial lipid levels to different levels in physically active men. Understanding these relevant differences will enable clinicians to provide the best exercise prescription for patients.
Postprandial lipemia (PPL) is characterized by an increase in triglyceride-rich lipoproteins in the plasma after a meal.1 Regular exposure to elevated PPL promotes plaque formation because of an increased infiltration of chylomicron remnants into the arterial wall and athrerogenesis.2 Patients with premature cardiovascular disease often have high PPL, which is a hidden risk factor found in some individuals with a normal lipid profile when measured after fasting.3 Despite the possible reversibility of abnormal PPL, the usual Western societal intake of three meals a day leads to metabolic disturbances after one meal that are unlikely to have adjusted prior to the next. As a result, up to two-thirds of the day may be spent in the postprandial state.4
Endurance exercise is known to reduce PPL, as seen in chronic and acute interventional studies.2,5,6,7 The benefits of aerobic exercises in acute PPL have been documented in several studies4,8,9, even when the exercise was completed 14 to 18 hours before a high-fat meal (HFM). Exercise may reduce PPL levels for up to eight hours after a meal.10 Several exercise variations have been tested, such as light, moderate and intense continuous and intermittent exercises.2,11,12 However, no studies have tested the effect of different exercise intensities on PPL while considering the participants' anaerobic threshold.
Thus, we hypothesize that exercise can attenuate PPL and produce a beneficial lipid profile. In some studies, the volume of exercise was not equivalent4,12,13, and the results led to some confusion about the best method to reduce PPL. The magnitude of the exercise-associated energy expenditure is of great importance when considering exercise-related PPL benefits. Therefore, the purpose of this study was to investigate the effect of intense intermittent versus moderate continuous exercise on postprandial lipemia while keeping the energy expenditure constant.
METHODSSubjectsTwenty healthy male volunteers between 20 to 40 years of age who were at least moderately active in accordance with the international physical activity questionnaire (IPAQ) were randomly selected (table 1). Other inclusion criteria were as follows: non-smokers, normal fasting lipid levels, free from disease, no neuropathies or orthopedic problems limiting physical tests and no concomitant medications influencing carbohydrate or lipid metabolism. The study was approved by the institutional Research Ethics Committee, and all volunteers signed a written consent form.
Subject characteristics.
| Variable | n = 20 | ||
|---|---|---|---|
| Age (yrs) | 21.5 | ± | 3.5 |
| Weight (kg) | 69.2 | ± | 9.0 |
| Height (m) | 1.74 | ± | 0.1 |
| BMI (kg/m2) | 23.0 | ± | 2.5 |
| Body fat (%) | 12.3 | ± | 2.5 |
| VO2 maximum (ml/kg/min-1) | 52.7 | ± | 7.9 |
| Anaerobic threshold (ml/kg/min-1) | 45.1 | ± | 6.7 |
| Anaerobic threshold (RPE) | 13.9 | ± | 2.2 |
| Triglycerides (mg/dL) | 100.5 | ± | 31.6 |
| Total cholesterol (mg/dL) | 148.0 | ± | 31.6 |
| HDL (mg/dL) | 42.9 | ± | 7.4 |
| LDL (mg/dL) | 84.2 | ± | 27.2 |
| VLDL (mg/dL) | 20.7 | ± | 5.6 |
| Insulin (μU/mL) | 14.1 | ± | 6.7 |
During a preliminary visit, subjects underwent a physical examination and had their weight, height, BMI, body composition, maximal oxygen uptake (VO2max) and anaerobic threshold (AT) assessed. These tests were performed at a temperature maintained between 21 and 23°C and a relative humidity between 40 and 60%.
VO2max was determined using a breath-by-breath method (Córtex - Metalyzer 3B from Córtex Biophysik – GER) during an incremental exercise test with 3-min stages and increments of 0.5 km/h. The initial velocity was set between 8 and 9 km/h on the treadmill (Brudden Equipamentos LTDA – Line Movement, model RT 400, São Paulo – BRA). The test was performed until voluntary exhaustion, reaching a blood pressure of ≥260/115 mmHg, rating of perceived exertion (RPE) >19 on the Borg 6-20 scale 14 or S-T segment depression or elevation greater than 2 mm on the electrocardiogram.15
During each test, the heart rate (HR) was measured continuously in addition to the blood pressure (BP), RPE, ventilation (VE), oxygen uptake (VO2), carbon dioxide release (VCO2), oxygen ventilation equivalent (VE/VO2), carbon dioxide equivalent VE/VCO2 and respiratory exchange ratio (RER). We considered VO2max to be valid when at least two of the following criteria were met: RER > 1.15, VO2 plateau Δ VO2 ≥ 150 ml in the last minute, HR < 10 bpm of 220 – age and/or RPE ≥ 19. HR was measured by a portable HR monitor belt (S-810i, Polar, Finland) and blood pressure by the auscultation method (Premium, Glicomed – Brazil).
AT was determined at the point where VE/VO2 increased disproportionately compared to the increase in VE/VCO2.16 Caloric expenditure was calculated according to the following equation: 3.9 × VO2 +1.1 × VCO2.17
For 48 hours prior to testing, each subject was instructed to avoid caffeine, alcohol, strenuous exercise and unusual foods in the daily intake while eating normally.
Study trialsAfter the maximal test, all subjects participated in three randomly selected trials on three different days with at least 48 h between sessions: control day (no exercise), CON-EX (moderate continuous exercise) and INT-EX (intense intermittent exercise). In the first experiment, the subjects recorded their food intake in the past 24 h and were asked to replicate the same food intake pattern (frequency and amounts) on the days preceding the tests.
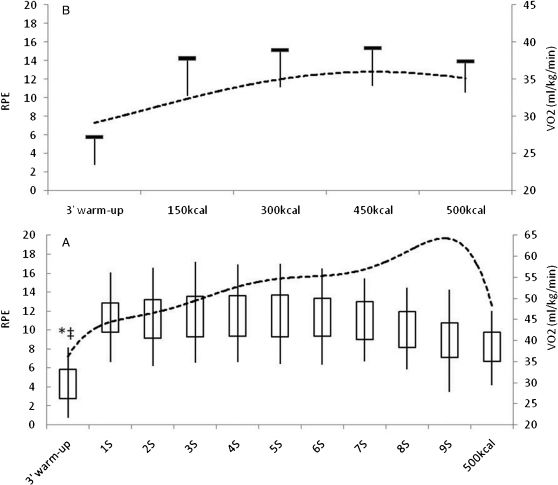
The CON-EX trial was by performed jogging continuously on a treadmill at a speed that was 85% of each individual AT; the INT-EX trial was conducted at 115% of the AT in 3-min sessions with 1.5 min of passive recovery. In both cases, participants expended 500 kcal of energy. Both trials began with a 3-min warm-up at 70% of the AT and lasted until the 500 kcal expenditure was reached, followed by a 3-min recovery activity at 70% anaerobic threshold. The energy expenditure was measured continuously; the energy generated in warm-up and recovery was computed as part of the 500 kcal. RPE was reported at the end of the warm-up, upon reaching 500 kcal, at every INT-EX test session, and upon reaching 150, 300 and 450 kcal on the CON-EX trials. When the 500 kcal expenditure was reached, subjects initiated a 3-min cool down procedure in which the treadmill speed was reduced to 5km/h for 1 min and 3 km/h for another 2 min (figure 1).
Means ± SD of VO2 and RPE during intermittent and continuous exercise. Figure 1A: dashed line - RPE; line at the bottom of the box – VO2 mean; line above box – VO2 peak; vertical line below the box – SD of VO2 mean; vertical line above the box – SD of VO2 peak; Figure 1B: dashed line – RPE; horizontal line – VO2 mean; vertical line – SD of VO2 mean. * Significant difference between VO2 mean from warm-up and each session in INT-EX; ‡ Significant difference between VO2 peak from warm-up and each session in INT-EX; # Significant difference between VO2 mean from warm-up and 150, 300, 450 and 500kcal energy expenditure measurement.
After the cool-down, the subjects sat for an additional 27 min. Gas analysis with caloric expenditure and average RER was performed during the exercise, cool-down and post-exercise rest period. After the break, subjects were given a high-fat meal. After the meal, gas analyses were repeated every hour for 10 min. On control day trials, subjects rested for 30 min and then consumed the same high-fat meal with the same subsequent measurements.
High-fat mealIn the three HFM trials, the subjects were instructed to consume the meal in approximately 15 min. Caloric ingestion was individually adjusted and contained 1.0 g of fat/kg of body mass, with ingredients added so that chocolate accounted for 20% of the lipid intake, Brazilian nuts for 20%, 10-min-boiled eggs for 18%, olive oil for 15% and milk powder for 10%. The total fat content corresponded to 71% of the energy provided. Specifically, the HFM was composed of 0.3 g of protein, 0.62 g of carbohydrate and 0.04 g of fiber per kg of body mass. Foods were added to 400 ml of water and blended for 5 min. The relative contributions of saturated, unsaturated and polyunsaturated fatty acids were 42.8%, 43.7% and 13.5%, respectively. No other food was provided for four hours after the HFM was ingested. Water was available in the postprandial period water ad libitum in the first trial, and the ingested amount was replicated in the following trials.
Blood analysisBlood samples were obtained through a venous puncture at baseline, immediately before the trials, and at 1, 2, 3 and 4 h postprandially. Serum triglyceride (TG), HDL cholesterol, total cholesterol and glucose were determined by enzymatic colorimetric methods using kits available commercially (Advia 2400, SIEMENS Helthcare Diagnostics Inc. Tarrytown, NY – USA, 2008). LDL and VLDL were estimated according to the following equation: VLDL = (triglyceride/5) and LDL = (total cholesterol – HDL – VLDL).18 Serum insulin was determined by fluoroimmunoassay using a commercially available kit (Immulite 2000, SIEMENS Helthcare Diagnostics Inc. Gwynedd, UK – Eng, 2009).
Calculations and statistical analysesThe total caloric expenditure (TCE) was estimated based on measurements made during the trials, post-exercise or control rest together with the postprandial calorie expenditure. The area under the variable curve was calculated using trapezoidal methods.9 The AUCs in the postprandial period are presented in mg/dL-1/4h-1 for total cholesterol, TG, HDL, LDL, VLDL and glucose variables, whereas insulin AUC is presented in μU/mL-1/4h-1.
Normality tests confirmed that all dependent variables of interest were normally distributed. Data are presented as the mean ± SD. The differences in the CON-EX and INT-EX trials were analyzed with a paired t-test. Repeated measures were analyzed using a variance analysis adjusted for covariance (MANCOVA) to examine the differences between each measurement in each trial (control, CON-EX and EX-INT). The baseline average of the three trials was adopted as a covariant. p<0.05 was considered significant. When significant overall differences between the trials were found, the data were further analyzed with an LSD post-hoc test. Statistical analyses were performed with SPSS for Windows version 11.5 (SPSS, Chicago, IL).
RESULTSSubjects' characteristicsThe HFM was well tolerated by all subjects. Table 1 documents the subjects' demographics and incremental and blood test performances, revealing that all blood parameters were within the range for normal healthy subjects. Subjects were moderately active according to the IPAQ questionnaire, and they exhibited a high level of fitness (52.7 ml/kg/min-1). The anaerobic threshold was identified at 85.0±4.4% of the VO2max, and a low percentage of body fat (12.3%) was observed.
Exercise sessionsSubjects exercised for a mean duration of 40 minutes during CON-EX and INT-EX. The peak VO2 during the exercise sessions for the CON-EX and INT-EX trials corresponded to 72.5% and 97.7%, respectively.
Table 2 presents the exercise volume and intensity, energy expenditure and RER obtained throughout the study.
Mean (±SD) exercise volume and intensity, energy expenditure and RER.
| Variables | Control | CON-EX | INT-EX | P |
|---|---|---|---|---|
| Distance (m) | - | 6815±983 | 6279±818* | 0.001 |
| Speed (km/hour) | - | 10.5±1.4 | 14.2±1.9* | 0.001 |
| Total time (min) | - | 39.8±3.9 | 40.3±4.4 | 0.292 |
| KCALexercise (kcal) | - | 501±2.2 | 498±17.1 | 0.369 |
| Mean of HR (bpm) | - | 160±10.9 | 170±11.9* | 0.036 |
| Peak of VO2 (ml/kg/min-1) | - | 38.2±5.1 | 51.5±7.2* | 0.001 |
| RPE (mean of test) | - | 12.4±2.6 | 15.0±2.0* | 0.001 |
| RER (mean of test) | - | 0.90±0.01 | 1.0±0.05* | 0.001 |
| KCAL30 (kcal) | 47.5±7.4† | 74.8±12.1 | 78.7±12.9 | 0.001 |
| KCALtotal (kcal) | 423±73.5† | 966±59 | 983±69 | 0.001 |
| RER30 | 0.85±0.01† | 0.80±0.1 | 0.80±0.1 | 0.046 |
| RER 4h | 0.79±0.01† | 0.74±0.01 | 0.74±0.01 | 0.001 |
Significant difference from CON-EX and INT-EX, p<0.05;
Legend: KCALexercise - energy expenditure for the year; HR – heart rate; VO2 – oxygen volume; RPE – rating of perceived exertion; KCAL30 – energy expenditure 30 minutes immediately after exercise or caloric expenditure control; KCALtotal – total energy expenditure throughout the experiment; RER30 – respiratory exchange ratio average 30 minutes immediately after exercise or control; RER 4h – respiratory exchange ratio average during the 4 hours postprandial.
As shown in Table 2, the running speed, RER, HR and VO2 in CON-EX were significantly lower than in INT-EX because the distance was greater in CON-EX. As expected, the main control variables (exercise time and energy expenditure) did not differ between experiments either during or after the exercise. Also as expected, these variables had significantly higher values on the test day than on the control day. The RERs for both INT-EX and CON-EX were lower during the 30-min period immediately after the trials and 4 h postprandial than on the control day.
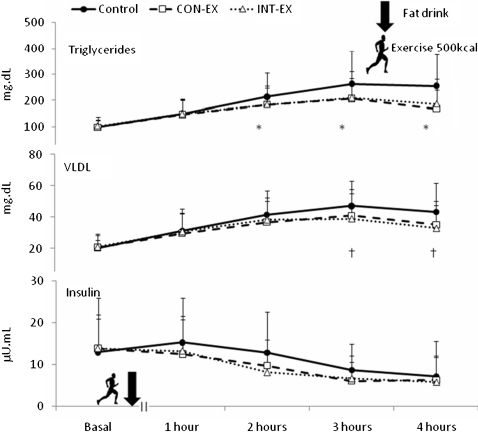
Postprandial valuesFigure 2 shows the TG, VLDL and insulin response over the three trials. MANCOVA results revealed a statistically significant trial × time interaction (p<0.05) for both TG and VLDL response. Post-hoc analyses indicated that the mean TG concentrations at 2, 3 and 4 h after INT-EX and CON-EX were lower than in the control trial (p<0.05). Accordingly, the mean VLDL concentrations at 2 and 4 h after INT-EX were lower (p<0.05); this was not true for the CON-EX. The mean values for insulin, total cholesterol and HDL and LDL cholesterols did not differ statistically across trials throughout the 4 h postprandial period.
The variable AUCs are shown in Table 3. In the control trial, the TG AUC was significantly increased by 18 and 15.4%, whereas the insulin AUC was significantly increased by 24.6 and 21.3% for CON-EX and INT-EX, respectively, compared with the control trial. The VLDL AUC was significantly increased by 12.5% in the control trial when compared with INT-EX (p<0.05) and tended to be increased by 11.4% in the control trial when compared with CON-EX (p = 0.06). Total cholesterol, HDL and LDL AUC values did not differ statistically between the trials. When comparing CON-EX versus INT-EX, no differences were found.
Area under the curve responses of lipemia variables and insulin obtained for the three trials.
| Variable | Control | CON-EX | INT-EX |
|---|---|---|---|
| AUC insulin (μU.mL-1.4h-1) | 61±35 * | 46±24 | 48±25 |
| AUC triglyceride (mg.dL-1.4h-1) | 993±388 * | 811±270 | 840±322 |
| AUC total cholesterol (mg.dL-1.4h-1) | 793±151 | 762±153 | 775±167 |
| AUC HDL (mg.dL-1.4h-1) | 218±39 | 210±31 | 210±38 |
| AUC LDL (mg.dL-1.4h-1) | 386±130 | 392±138 | 394±131 |
| AUC VLDL (mg.dL-1.4h-1) | 184±60† | 163±55 | 161±54 |
Some studies have examined the influence of resistance2,7 or acute endurance exercise5,6,19–21 on PPL. However, to date, no study has examined PPL associated with continuous aerobic or intermittent exercise according to the individual AT.
The major findings of this study show that CON-EX and INT-EX 30 min before HFM ingestion were effective in reducing TG AUC, insulin AUC and TG values from the second to the fourth hour. In addition, INT-EX reduced VLDL values in the third and fourth hour and the total VLDL AUC. No difference was found between CON-EX and INT-EX. As seen in Table 2, control variables of volume and intensity for the two trials, such as velocity, distance and RPE, differ from each other. However, energy expenditure did not differ between experiments after trials were equalized. Therefore, the differences in this study resulted from the different exercise intensities, in agreement with Katsanos et al. 2004,12 who found that the PPL response was directly associated with energy expenditure.
These data contradict findings reported by Teixeira et al22 and Tyldum et al,23 who analyzed the effect of intermittent and continuous exercise and found no reduction in PPL after HFM. Although these studies did not describe the caloric expenditure during trials, we assume that none reached the level of energy expenditure used in our study. Another study24 with energy expenditures of 110, 157 and 203 kcal did not find a reduction of TG levels in the postprandial period. The low level of caloric expenditure during exercise was associated with the RER value and indicated fat oxidation similar to that found on the control day, which led to the absence of PPL reduction.
Our data corroborate findings reported by Miyashita et al,25 who investigated intermittent and continuous exercise and found that both types of exercise sessions decreased PPL. In their study, the exercise intensity and volume were the same and the only change was the exercise method (continuous vs. intermittent). Our study tested different intensities (15% below and 15% above the speed corresponding to the AT). Similarly, other authors4,26 suggested that PPL decreases do not depend on the intensity when energy expenditures are the same.
PPL was attenuated when moderate-intensity exercises (65% VO2 peak) ended 1 hour before HFM, but not when light-intensity exercise (25% VO2 peak) and the same energy expenditure were used.12 The increased activity of the LPL enzyme might be mediated by the lower release of insulin as a result of moderate, not light, exercise. LPL facilitates the clearance of TG from plasma into muscle to replace intramuscular TG oxidized during exercise.24,27
The mechanism of the association between the insulin response and the intensity of exercise is not yet understood. However, evidence suggests that muscle glycogen contributes substantially to total energy production during intense exercise, whereas its contribution during light exercise is minimal. Therefore, the decrease of muscle glycogen in intense exercise increases the GLUT-4 content on the muscle cell surface, which increases glucose transport into the cell and consequently reduces requirements for the disposal of glucose mediated by insulin.28
The association between exercise, increased LPL activity and the consequent PPL response mediated particularly by insulin should be further evaluated. In our study, CON-EX and INT-EX had similar reductions in insulin AUC. However, INT-EX reduced VLDL, VLDL AUC, and TG in the last two hours, whereas only TG was reduced in CON-EX. Barrett et al.4 reported similar PPL responses, although the insulin values differed. Therefore, another mechanism mediates PLP and other factors in addition to insulin action and LPL acting on PPL.29 However, fatty acids derived from adipose tissue may still contribute to a greater formation of VLDL in the liver, particularly in CON-EX. In INT-EX the perfusion of adipose tissue may be compromised, and the fat pathway may be underutilized; during CON-EX a substantial amount of adipose tissue lipids may produce energy during exercise and later lead to an increase in the production of serum VLDL.
Energy expenditures during exercise appear to be important determinants of the extent to which PPL is reduced, which agrees with previous studies.26,27,30 Zhang et al.13 compared different volumes with the same exercise intensity and found reduced PPL when compared with the resting condition. In our study, energy expenditure during exercise and 30 min after exercise as well as the total energy expenditure were significantly higher in the INT-EX and CON-EX trials when compared to the control trial.
Intermittent exercise and energy expenditures of 245 kcal (30 min) reduced the TG values in the postprandial period, whereas continuous exercise did not.11 This difference may be explained by the increase in LPL activity and excess post-exercise oxygen consumption (EPOC) during intermittent, but not continuous, exercise. In our study, the EPOC did not differ between trials conducted at 85 and 115% of AT. However, the PPL response was better in INT-EX than in CON-EX.
RER may be another mechanism for explaining PPL reduction after exercise.24 In our study, the RER was measured immediately after exercise, after half an hour and four hours after ingesting a HFM, which led to higher fat oxidation during CON-EX and INT-EX; this higher oxidation was not seen in the control trials. This oxidation would facilitate the clearance of TG from plasma into muscle to replace the intramuscular TG oxidized during exercise and lipids used by the liver for neoglucogenesis to replace the glycogen depleted during exercise.27
RER may also explain the PPL reduction after exercise.24 In this study, the RER immediately after exercise and half an hour and four hours after ingesting HFM showed higher fat oxidation during CON-EX and INT-EX than during the control trials. As described above, this would facilitate the clearance of TG from plasma into muscle.27 Decreased post-exercise RER, which suggests high relative fat oxidation rates, high energy expenditures resulting from increased oxygen uptake during exercise and EPOC contributed to high TG clearance rates in the postprandial period in exercise trials; these reductions were not seen in the control trial.7
In addition, reduced synthesis and secretion of VLDL-triglycerides in the liver during the postprandial period may be associated with exercise and PPL attenuation, as such reductions were not observed in the control trial. Fatty acid oxidation in the liver was elevated, which reduced the availability of TG for incorporation into VLDL.25 Other authors10 suggest that when exercise is concluded immediately before HFM ingestion, the reduction in the hepatic secretion of triglyceride-rich VLDL may be more important to reduce PPL than the increase in LPL activity. Moreover, the LPL activation peak occurs four to eight hours after exercise. In the first hours after exercise, PPL reduction may also result from the reduction of chylomicrons in the HFM.
Reduced synthesis and secretion of VLDL-triglycerides in the liver may be associated with the mechanism described earlier, but our findings are not sufficient to confirm this association.
Although the changes in PPL metabolism are transient, the intake of an excessive HFM (1 g/kg) increased lipid values for four hours after ingestion. The metabolic events associated with a meal may persist until another meal is consumed, and people may have postprandial hyperlipemia during most of the day. As this may lead to the development of atherosclerosis and CVD, strategies to attenuate the magnitude and duration of PPL should be developed.
Physical activity is an important tool to prevent postprandial hyperlipidemia. Although both continuous moderate and intense intermittent exercises were effective in the attenuation of postprandial hypertriglyceridemia and hyperinsulinemia, only intense exercise reduced VLDL. This information is particularly important for physical activity recommendations if the intervention is aimed at controlling dysbetalipoproteinemic phenotypes.31
One of the limitations of this study was the recall bias of self-report in the first trial and the failure to comply in the other pre-trials. The short postprandial time (4 hours) also was a limitation because changes may affect profiles beyond that time. However, an abbreviated 4-hour PPL test seems to be a valid surrogate for an 8-hour test.32 Finally, variations in genes such as those encoding the apolipoproteins A, B and E may directly affect PPL due to their involvement in lipid metabolism.31,33 Therefore, studies using longer postprandial times and examining allelic variations should be conducted to better understand the different PPL mechanisms.
Our results suggest that intense intermittent or moderate continuous exercise and an energy expenditure of 500 calories for 30 min before ingestion of HFM reduces the PPL phenotype in physically active men. As individuals in western countries primarily exist in a postprandial state, our findings support the prescription of moderate and intense exercises because of their protective effects upon lipid metabolism.