The aim of this study was to investigate the protective effects of aqueous extracts of roselle (Hibiscus sabdariffa L. UKMR-2) against red blood cell (RBC) membrane oxidative stress in rats with streptozotocin-induced diabetes.
METHODS:Forty male Sprague-Dawley rats weighing 230-250 g were randomly divided into four groups (n = 10 rats each): control group (N), roselle-treated control group, diabetic group, and roselle-treated diabetic group. Roselle was administered by force-feeding with aqueous extracts of roselle (100 mg/kg body weight) for 28 days.
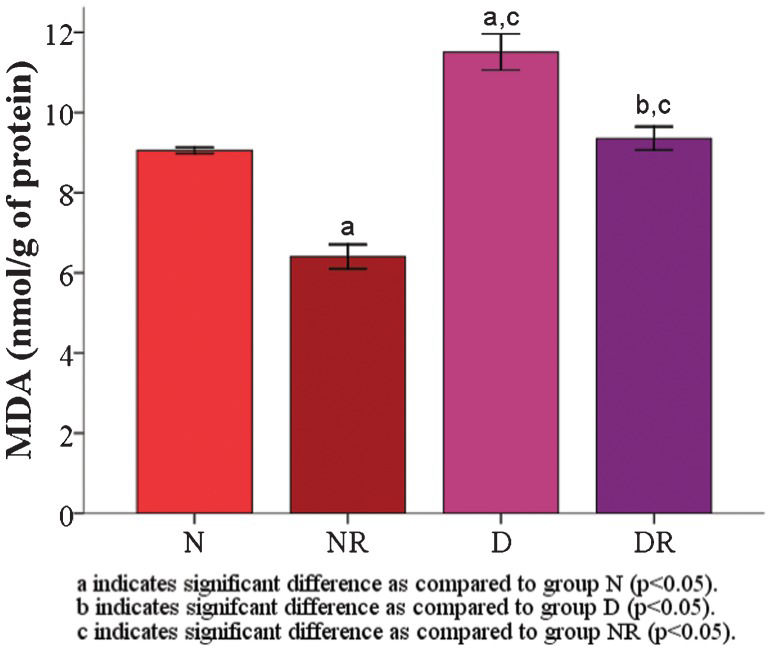
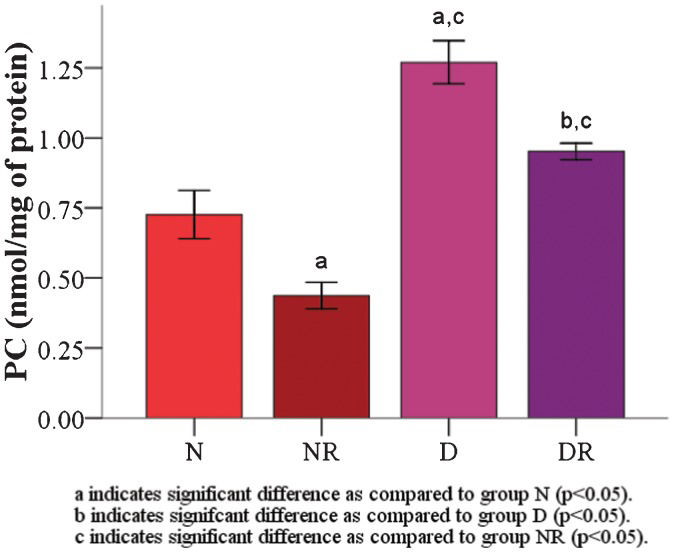
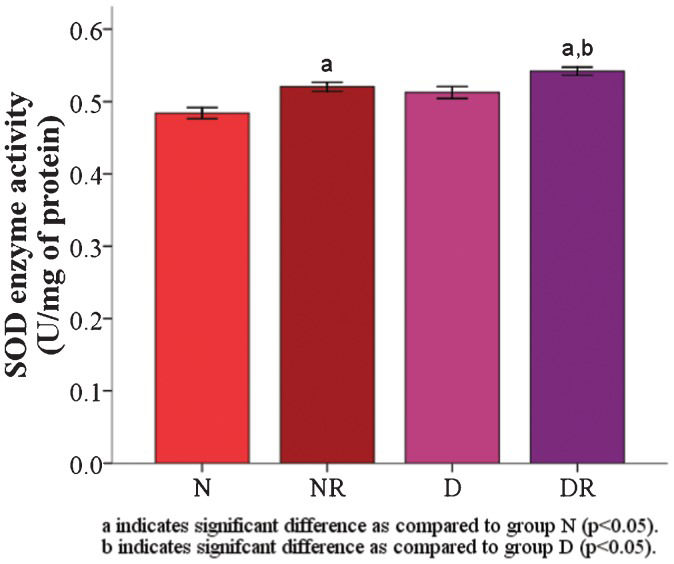
RESULTS:The results demonstrated that the malondialdehyde levels of the red blood cell membranes in the diabetic group were significantly higher than the levels in the roselle-treated control and roselle-treated diabetic groups. The protein carbonyl level was significantly higher in the roselle-treated diabetic group than in the roselle-treated control group but lower than that in the diabetic group. A significant increase in the red blood cell membrane superoxide dismutase enzyme was found in roselle-treated diabetic rats compared with roselle-treated control rats and diabetic rats. The total protein level of the red blood cell membrane, osmotic fragility, and red blood cell morphology were maintained.
CONCLUSION:The present study demonstrates that aqueous extracts of roselle possess a protective effect against red blood cell membrane oxidative stress in rats with streptozotocin-induced diabetes. These data suggest that roselle can be used as a natural antioxidative supplement in the prevention of oxidative damage in diabetic patients.
Diabetes mellitus is associated with oxidative stress as a result of increased free radical formation, such as the superoxide (O2•-) and hydroxyl (•OH) free radicals, and decreased activity of antioxidant defense systems (1). Hyperglycemia increases the formation of reactive oxygen species (ROS) via several pathways, such as glucose autoxidation (2), the polyol pathway (3), and non-enzymatic protein glycation (4). Both the polyol pathway and the protein glycation process favor the generation of advanced glycation end products (AGEs), which further worsen oxidative stress (5). The process of glycolysis and the pentose phosphate pathway have also been shown to contribute to the formation of ROS in patients with diabetes (1).
Red blood cells (RBCs) are susceptible to attacks by ROS because of their high polyunsaturated fatty acid (PUFA) content and their abundance of iron (Fe2+)-rich hemoglobin (5). Ionic Fe2+ acts as a catalyst in redox reactions and lipid peroxidation and forms malondialdehyde (MDA) as the end product (6). In addition, in diabetes, RBCs often undergo membrane protein oxidation or carbonylation (7). Therefore, protein carbonyls are common indicators of oxidative damage to proteins in cells (7). Diabetes mellitus also weakens the components of antioxidant defense systems, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (1). As a result of the oxidative attack on RBC membrane lipids, membrane proteins, and cytoskeletal proteins, the structure and function of the membrane bilayer may change, which further damages the RBC membrane as indicated by increased osmotic fragility and alterations in RBC morphology (8).
Roselle (Hibiscus sabdariffa L.) is a tropical annual herbal shrub that belongs to the Malvaceae family and is characterized by red calyces and flowers with a unique sour taste. Roselle calyces have been widely used as an edible colorant in food and drink preparations and are rich in antioxidant components, mainly anthocyanin, that counteracts oxidative damage to prevent diseases (9,10). Previous studies have shown that the free radical scavenging effect of Hibiscus sabdariffa extract is able to attenuate lipid peroxidation and protein oxidation in hepatic and renal tissues (11).
Hibiscus sabdariffa L. UKMR-2 is cultivated by Universiti Kebangsaan Malaysia, Malaysia, and has been shown to possess a higher anthocyanin content than other local varieties, e.g., UKMR-1 and UKMR-3 (12). The aim of this study was to investigate the antioxidant effect of roselle aqueous extracts (Hibiscus sabdariffa L. variety UKMR-2) on cellular plasma membrane oxidative stress in rats with streptozotocin (STZ)-induced diabetes. The RBC membrane was chosen as a representative model of cell membrane function because RBCs lack membranes derived from organelles (8). STZ was used to induce type 1 diabetes mellitus because it selectively destroys pancreatic beta cells (13).
MATERIALS AND METHODSPreparation of roselle aqueous extractsRoselle (H. sabdariffa L. UKMR-2) calyces were obtained from the Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Malaysia. Calyces were added to distilled water in a 1:2 ratio and ground in a blender (Cornell, Petaling Jaya, Malaysia) for 10 minutes. Next, the roselle extract was boiled until air bubbles appeared. The boiled extract was then cooled and filtered. The roselle extract was stored in an aluminum-covered bottle at 4°C. Extracts that needed to be stored for a longer period were freeze-dried (Labconco Co., Missouri, USA) and could be used for approximately 6 months.
Experimental animalsForty male Sprague-Dawley rats weighing 230-250 g were obtained from the animal facility of the Institute of Medical Research, Kuala Lumpur, Malaysia. All animal experimental protocols were performed in accordance with the guidelines issued by the Universiti Kebangsaan Malaysia Animal Ethics Committee (FSK/BIOMED/2011/SATIRAH/30-NOVEMBER/407-NOVEMBER-2011-MAY-2012). Rats were housed in clean cages and fed with rat chow and water throughout the experimental period (28 days). Rats were randomly divided into 4 groups of 10 rats each: control (N), roselle-treated control (NR), diabetic (D), and roselle-treated diabetic (DR) rats.
Induction of diabetesFollowing an overnight fast, diabetes was induced in groups D and DR by intravenous injection of STZ (Sigma Chemicals, St. Louis, Missouri, USA) (freshly dissolved in 0.9% normal saline before use) at a dose of 45 mg/kg body weight into the tail vein of the rats (14). The fasting blood glucose level was measured after 3 days to assess the development of diabetes mellitus.
Treatment groupsRoselle was administered to the NR and DR groups by force-feeding with aqueous extracts of roselle (100 mg/kg body weight) (15,16) for 28 days. Groups N and D were given distilled water at a dose of 1 ml/kg body weight in the same manner and for the same duration.
Blood sample collectionFollowing the experimental period (28 days), rats were again fasted overnight and blood was then drawn under light ether anesthesia from the sinus orbital or by cardiac puncture. To conduct the osmotic fragility test, blood smear preparation, and ghost membrane preparation, fresh blood was collected in EDTA tubes and placed in an ice bath. The blood was centrifuged (3,500 rpm, 5 minutes, 4°C) to separate the packed RBCs from the plasma and buffy coat. The packed RBCs were then washed three times in 0.9% normal saline. Afterward, the packed RBCs were suspended in 0.9% normal saline (ratio 1:1). Then, 50% of the RBC suspensions were aliquoted and stored at -40°C for future use.
RBC ghost membrane preparationRBC ghost membranes were prepared according to the method described by Dodge et al. (17). A volume of 50% RBC suspension was added to 10 ml of cold isotonic phosphate-buffered saline (PBS) at a pH of 7.4 and left for 30 minutes at 4°C. The suspension was then centrifuged (4,000 x g, 20 minutes, 4°C), and the supernatant was decanted. Sedimented cells were then resuspended in cold 1.25 mM PBS at a pH of 7.4 (ratio 1:15) and left for 30 minutes at 4°C. The suspension was then centrifuged four times (20,000 x g, 40 minutes) (Eppendorf AG, Hamburg, Germany), and the supernatant was decanted. RBC ghosts were then resuspended in an equal volume of cold PBS and kept at -40°C before use.
Determination of total RBC membrane proteinThe total RBC membrane protein was determined using 100-μl suspensions of RBC ghosts according to the method described by Bradford (1976), using Coomassie Brilliant Blue G-250 (Acros Organics, New Jersey, USA) at 595 nm (SI Analytics GmbH, Hattenbergstraβe 10, Mainz, Germany) (18). The results are expressed as mg/ml.
Determination of RBC membrane malondialdehyde (MDA)RBC membrane MDA was determined as a measure of lipid peroxidation in 0.1 ml of RBC ghosts according to the method described by Stocks and Dormandy (19), using the thiobarbituric acid reagent (ICN Biomedicals, Irvin, California, USA) at 100°C. The pink adducts that formed were extracted in butanol (Fisher Scientific, Massachusettes, USA) and measured spectrophotometrically at 532 nm (19). The results were expressed as nmol/g of protein.
Determination of RBC membrane protein carbonyl (PC)According to the method described by Levine et al. (20), RBC membrane PC was measured spectrophotometrically at 360 nm as an index of protein oxidation in 50 μl of ghosts, using the 2,4-dinitrophenylhydrazine reagent (Sigma Chemicals, St Louis, Missouri, USA) to form protein-conjugated hydrazones (20). The results were expressed as nmol/mg of protein.
Assay of RBC membrane superoxide dismutase (SOD) enzyme activityThe RBC membrane SOD enzyme activity was determined according to the method described by Beyer and Fridovich (21) with slight modifications. The following reaction mixture was made in the dark: 20 μl of ghosts, 27 ml of phosphate buffer at a pH of 7.8, 1.5 ml of 30 mg/ml L-methionine (Acros Organics, New Jersey, USA), 1 ml of 1.41 mg/ml nitro blue tetrazolium chloride (Acros Organics, New Jersey, USA), and 0.75 ml of 1% Triton X-100 (Fisher Scientific, Massachusettes, USA). The mixture was added to 10 μl of riboflavin (Acros Organics, New Jersey, USA). The reaction mixture was then exposed to Sylvania arolux fluorescent light (18 W) for 7 minutes and measured spectrophotometrically at 560 nm. The results were expressed as U/mg of protein.
Determination of osmotic fragilityRBC osmotic fragility was determined according to the method described by Dacie and Lewis (22). A total of 0.05 ml of fresh blood was added to increasing concentrations of a sodium chloride (NaCl) solution (0, 0.30, 0.45, 0.55, and 0.85%), gently mixed, and incubated at room temperature (25°C) for 30 minutes. The mixtures were then centrifuged (2,000 x g, 5 minutes) and measured spectrophotometrically at 540 nm. The results were expressed as the percentage (%) of hemolysis.
RBC morphologyBlood smears were prepared according to the method described by Dacie and Lewis (22), and staining was performed according to the method described by Wright (23), with slight modifications. The blood smears were prepared with one drop of fresh blood followed by Wright's staining (Sigma Chemicals, St Louis, Missouri, USA). The stained blood smears were visualized using a light microscope.
Statistical analysisAll data were tested for normality using the Shapiro-Wilk test (p>0.05), and Levene's test was used to assess homogeneity (p>0.05). Using SPSS Version 18 (Armonk, New York, USA), four groups were compared using one-way analysis of variance (ANOVA) followed by the Bonferroni (post hoc) test. Differences were statistically significant at p<0.05. The results are expressed as the mean±standard error of the mean (SEM).
RESULTSAnimalsThe mean body weights of all four groups were similar at the beginning of the study (246.25±7.43 g). After the induction of diabetes, all 20 rats were used for the following 28-day period. Body weight, blood glucose, and food and water intake were monitored in all of the studied animals (Table 1). At the end of the experiment, both diabetic groups demonstrated weight loss and a significant increase in their blood glucose levels (p<0.05). However, roselle treatment in the DR group resulted in a significant decrease in the blood glucose level compared with the DA group (p<0.05). Food and water intake were significantly increased in both diabetic groups compared with the non-diabetic controls (p<0.05). Overall, roselle treatment did not affect any of the above parameters.
Body weight, blood glucose, and food and water intake.
| Group N | Group NR | Group D | Group DR | |
|---|---|---|---|---|
| Body weight (g) | 318.63±10.40 | 328.88±10.42 | 233.44±15.28ab | 206.40±14.58ab |
| Blood glucose (mmol/L) | 6.8±0.80 | 6.1±0.60 | 26.85±1.33ab | 19.32±1.48abc |
| Food intake (g) | 4.07±0.15 | 6.14±0.75 | 14.73±0.40ab | 13.45±0.60ab |
| Water intake (mL) | 8.73±2.09 | 8.95±0.90 | 44.52±2.55ab | 43.21±2.35ab |
Values are expressed as the mean±SEM (n = 8 per group).
a indicates a significant difference compared with group N (p<0.05).
b indicates a significant difference compared with group NR (p<0.05).
c indicates a significant difference compared with group D (p<0.05).
The RBC membrane malondialdehyde (MDA) level (11.51±0.45 nmol/g) and protein carbonyl (PC) level (1.27±0.08 nmol/g) were significantly increased (p<0.05) compared with group N (MDA = 9.06±0.07 and PC = 0.73±0.09 nmol/g) and group NR (MDA = 6.41±0.30 and PC = 0.44±0.05 nmol/g) (Figures 1 and 2). Both the RBC membrane MDA (Figure 1) and the PC (Figure 2) levels (MDA and PC levels for group DR were 9.35±0.29 and 0.95±0.03 nmol/g, respectively) were found to be significantly decreased in the two roselle-treated groups compared with groups N and D (p<0.05).
There was no significant difference (p>0.05) in the total protein level between the groups (N: 49.33±1.78 mg/ml; NR: 48.60±3.11 mg/ml; D: 44.38±3.82 mg/ml; DR: 44.70±2.72 mg/ml).
The RBC membrane SOD enzyme activity was significantly higher (p<0.05) in the roselle-treated groups (NR = 0.52±0.01 and DR = 0.54±0.01) than in the groups that were not treated with roselle (Figure 3). The RBC osmotic fragility in group D was slightly higher than that in group N but was not significantly different (Table 2).
Values of osmotic fragility for the control group (N) and the STZ-induced diabetes group (D) following 28 days of treatment with aqueous extracts of roselle.
| NaCl (%) | Group N | Group NR | Group D | Group DR |
|---|---|---|---|---|
| 0.85 | 0 | 0 | 0 | 0 |
| 0.55 | 1.41±0.33 | 1.31±0.34 | 3.96±2.44 | 1.20±0.34 |
| 0.45 | 22.91±6.88 | 37.55±6.66 | 37.65±7.44 | 40.05±7.03 |
| 0.30 | 92.51±1.84 | 82.75±3.05a) | 94.44±0.98 | 89.53±1.98 |
| 0.00 | 100 | 100 | 100 | 100 |
Values are expressed as the mean±SEM.
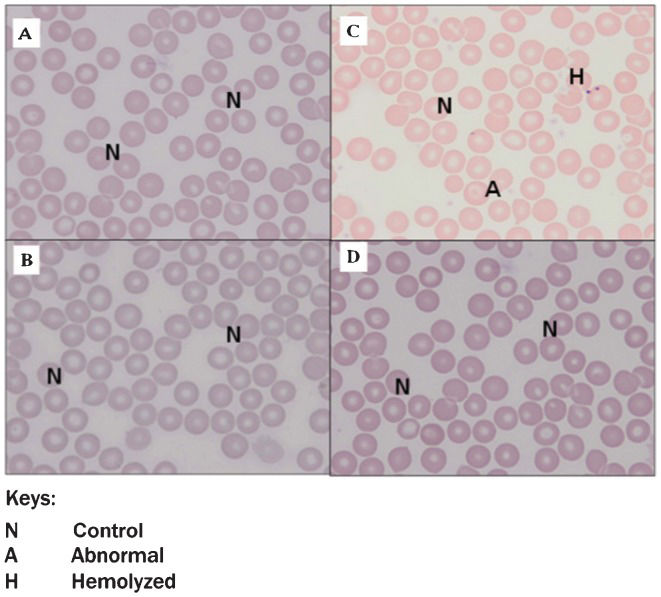
The RBC morphology of both roselle-treated groups (Figure 4B) and Figure 4D was normal (Figure 4A). There were also no significant morphological changes in the RBCs of the diabetic rats compared with the RBCs of the control rats. However, there was the presence of a small number of abnormal (A) or hemolyzed (H) RBCs in the blood smears of diabetic rats (Figure 4C).
DISCUSSIONThe aim of the present study was to evaluate the protective effects of a newly mutated variety of H. sabdariffa L. UKMR-2 on RBC oxidative stress in diabetic rats. UKMR-2 was chosen because it has the most potential for commercialization and contains the highest anthocyanin content among the three H. sabdariffa variants (12). In the present study, we observed significant decreases in the RBC membrane MDA and PC levels, as well as a significant increase in the RBC membrane SOD enzyme activity.
In the present study, the total RBC membrane protein level in diabetic rats was not significantly different from that of the other groups, which was consistent with the results found in previous studies (24,25). The total protein level is not a specific marker of protein damage; however, PCs play a role as essential oxidative markers of RBC membrane proteins (7).
The significant increase in the RBC membrane PC level in diabetic rats observed in this study suggests that diabetes induces an increased level of free oxygen radicals and resultant oxidative protein damage, which is supported by the observation that increased blood glucose levels were associated with a high RBC membrane PC content in diabetic patients (26). A possible mechanism of PC production during oxidative stress is the direct oxidation of proline, arginine, and lysine residues (27).
In addition to RBC membrane protein damage, free radicals also attack RBC membrane lipids, especially PUFAs, leading to lipid peroxidation. MDAs were significantly increased in the RBC membranes of diabetic rats, which is consistent with the results of previous studies (28,29). In contrast, SOD activity in RBC membranes was higher in diabetic rats than in control rats (30), which could be due to the activation of an antioxidant defense system to suppress the formation of free radicals (25).
Both RBC membrane MDA and PC levels were significantly reduced in the two roselle-treated groups (NR and DR). A previous study showed that roselle extracts significantly decreased MDA formation in the hepatic tissue of diabetic rats and in the linoleic acid oxidation system (11). In control rats treated with roselle extracts, a reduced MDA level was also observed in the renal tissue (35). It has also been demonstrated that roselle extracts effectively reduce protein oxidation in the hepatic and renal tissues of diabetic rats. Furthermore, a tendency toward decreased PC levels was observed in the hepatic and renal tissues of control rats treated with roselle extracts (11).
However, the slight increase in SOD enzyme activity could not compensate for the increase in free radicals, as demonstrated by the increase in PC and MDA levels. These changes may cause damage to the membrane protein structure and lipid (PUFA) content. As a result, the intrinsic membrane mechanical properties are altered, resulting in reduced deformability and reduced fluidity or altered permeability of the phospholipid bilayer, which, in turn, reduces the ability of the membrane to withstand osmotic changes (32).
The present study also demonstrated that there were no significant changes in osmotic fragility or RBC morphology in roselle-treated rats (NR and DR). The roselle extract had a similar effect on the osmotic fragility of human RBCs (31). The results of the present study suggest that the roselle extract has a protective effect on the RBC membrane against free radical attacks that allows the RBCs to maintain normal morphology in diabetes. In addition, it has also been found that anthocyanins are able to localize to the plasma membrane (36); therefore, they can further protect the membrane from the oxidative attacks of free radicals. The structure of anthocyanins consists of an o-diphenol structure in ring-B and a conjugated double bond system. This structure gives the molecule the ability to scavenge radicals by hydrogen donation, to assist in radical stabilization, (33) to protect RBCs against ROS attacks, and to increase RBC integrity and function (34).
In conclusion, the aqueous extract of roselle (Hibiscus sabdariffa L. UKMR-2) possesses antioxidant properties, as demonstrated by its protective effect against RBC membrane oxidative stress in rats with STZ-induced diabetes.
AUTHOR CONTRIBUTIONSMohamed J was the supervisor. Shing SW and Idris MH performed the experiments. Budin SB was the co-supervisor. Zainalabidin S wrote the manuscript.
We thank Sabarina Ismail for her assistance in the preparation of the biochemical tests. This study was supported by a grant from Universiti Kebangsaan Malaysia (UKM-GUP-2011-124).
No potential conflict of interest was reported.