Accelerated bone loss that occurs in postmenopausal women has been linked to oxidative stress and increased free radicals. We propose the use of antioxidants to prevent and reverse postmenopausal osteoporosis. This study aimed to examine the effects of tocotrienol, a vitamin E analog, on bone loss due to estrogen deficiency. Our previous study showed that tocotrienol increased the trabecular bone volume and trabecular number in ovariectomized rats. In the current study, we investigated the effects of tocotrienol supplementation on various biochemical parameters in a postmenopausal osteoporosis rat model.
MATERIALS AND METHODS:A total of 32 female Wistar rats were randomly divided into four groups. The baseline group was sacrificed at the start of the study, and another group was sham operated. The remaining rats were ovariectomized and either given olive oil as a vehicle or treated with tocotrienol at a dose of 60 mg/kg body weight. After four weeks of treatment, blood was withdrawn for the measurement of interleukin-1 (IL1) and interleukin-6 (IL6) (bone resorbing cytokines), serum osteocalcin (a bone formation marker) and pyridinoline (a bone resorption marker).
RESULTS:Tocotrienol supplementation in ovariectomized rats significantly reduced the levels of osteocalcin, IL1 and IL6. However, it did not alter the serum pyridinoline level.
CONCLUSION:Tocotrienol prevented osteoporotic bone loss by reducing the high bone turnover rate associated with estrogen deficiency. Therefore, tocotrienol has the potential to be used as an anti-osteoporotic agent in postmenopausal women.
To maintain its overall health and function, bone continuously undergoes a regeneration process known as bone remodeling. The process involves bone resorption by osteoclasts followed by formation of new bone by osteoblasts. Bone resorption and formation are closely coupled such that the amount of bone destroyed by osteoclasts is equal to the amount of bone formed by osteoblasts. The molecular basis of the coupling process in bone remodeling involves cytokines such as macrophage-colony stimulating factor (M-CSF), receptor activator of nuclear factor kappa b ligand (RANKL) and osteoprotegerin (OPG). The binding of M-CSF and RANKL to their respective surface receptors on osteoclast precursors enables them to differentiate into mature multinucleated osteoclast cells. This process is regulated by osteoprotegerin, which competes with RANKL to inhibit osteoclast formation (1,2). Meanwhile, bone resorption causes the release of osteoclast-derived ‘coupling factors’, which are embedded in the bone matrix by osteoblasts during bone formation. These coupling factors include growth factors, such as transforming growth factor beta (TGF-beta), bone morphogenetic proteins and insulin-like growth factor (IGF), as well as factors produced and released by osteoclasts, including cardiotropin-1, which stimulate osteoblast differentiation and bone formation (2).
In postmenopausal osteoporosis, estrogen deficiency leads to loss of bone, rendering it susceptible to fracture. The mechanisms for bone loss include RANKL upregulation, which leads to increased osteoclast recruitment and activation; decreased osteoclast apoptosis; reduced osteoprotegerin production by osteoblasts, causing an increase in the RANKL/osteoprotegerin ratio that favors bone resorption; increased expression of bone-resorbing cytokines, such as M-CSF, tumor necrosis factor-α (TNF-α), interleukin-1 and interleukin-6; and a direct effect on osteoclasts via the inhibition of apoptosis and an increase in the differentiation of osteoclast precursors into mature osteoclasts (3).
Reactive oxygen species have widely been considered to be a causal factor in a number of pathological conditions, including osteoporosis. Studies by Bax et al. (4) and Garrett et al. (5) reported that osteoclast differentiation and functions were stimulated by reactive oxygen species. Another study showed that estrogen deficiency lowered antioxidant defenses in osteoclasts, resulting in increased osteoclastic resorption (6). Several of the intracellular signals essential for osteoclast formation, such as nuclear factor-kappa B (NF-κB), c-Jun amino-terminal kinase (JNK) and phosphatidylinositol 3-kinase (PI3K) are sensitive to reactive oxygen species (7).
Previous researchers have reported that antioxidant vitamins can effectively reduce oxidative damage both in vitro and in vivo (6,8). In the present study, we determined the effects of vitamin E in the form of tocotrienol on bone metabolism in ovariectomized rats by analyzing the biomarkers of bone turnover.
MATERIALS AND METHODSAnimalsA total of 32 female Wistar rats (three months old), weighing 160-190 g, were obtained from the Animal House of the Universiti Kebangsaan Malaysia. The rats were equally divided at random into four groups with eight rats in each group. The baseline group was sacrificed at the start of experiment, and a second group was sham operated. The remaining rats were ovariectomized and either given olive oil or treated with tocotrienol at a dose of 60 mg/kg body weight. The sham rats were also given olive oil. Treatment commenced two weeks after ovariectomy to allow the rats to recuperate. Olive oil or tocotrienol was given orally to the rats using an oral gavage needle six days per week for four weeks.
The rats were housed in standard cages in groups of three at room temperature with a 12 h light-dark cycle. The animals were fed with a commercial rat chow diet (Gold Coin, Klang, Selangor, Malaysia), and tap water was provided ad libitum. Daily food intakes and weekly body weights were documented.
Pure tocotrienolThe tocotrienol mixture was prepared by the Palm Oil Research Institute of Malaysia (PORIM; Malaysian Palm Oil Board, Kajang, Selangor, Malaysia) and had the following composition: 37.2% alpha-tocotrienol, 39.1% gamma-tocotrienol and 22.6% delta-tocotrienol. The palm tocotrienol mixture was diluted in olive oil (Bertolli Classico, Italy) to obtain a concentration of 60 mg/kg body weight. The dose chosen was based on our previous study, which showed that at a dose of 60 mg/kg body weight, tocotrienol was able to prevent the oxidative stress-induced increase in interleukin-1, a bone resorbing cytokine, in a rat model (9). This dose is equivalent to 6 mg/kg in humans, or approximately 420 mg for a 70 kg human, after adjusting for differences in surface area (10).
Specimen collectionThe rats were sacrificed by cervical dislocation under anesthesia with ether. Subsequently, their abdomens were dissected to expose their hearts for blood sampling via cardiac puncture. The serum was separated and stored in a deep freezer at a temperature of -70°C.
Serum biochemistrySerum levels of interleukin-1, interleukin-6, osteocalcin and pyridinoline were measured at the end of the experiment using the enzyme immunoassay (EIA) technique. The results obtained with commercially produced rat enzyme immunoassay kits for interleukin-1 (Biosource International, Camarillo, California, USA), interleukin-6 (BenderMed Systems, Vienna, Austria), osteocalcin (Biomedical Technologies, Stoughton, Massachusetts, USA) and pyridinoline (Quidel Corp., San Diego, California, USA) were analyzed using an enzyme immunoassay reader (VERSAmax, ELISA Reader, Molecular Devices LLC, Sunnyvale, California, USA).
Statistical analysisThe results are expressed as the mean±SEM. Data analyses were performed using Statistical Package for Social Sciences software (SPSS, USA). Normality was tested with the Kolmogorov-Smirnov test. Data that were found to be normally distributed were subjected to ANOVA followed by Tukey's HSD post-hoc analysis. The significance level was set at p≤0.05.
All the protocols used in this study were approved by the Animal Ethics Committee of the Universiti Kebangsaan Malaysia (approval number FAR/IMA/23-JULY/075).
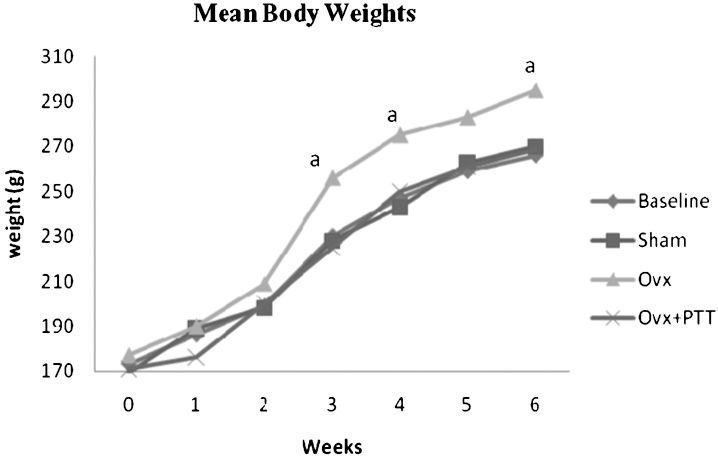
RESULTSBody weights and food intakeThere was no significant difference in the mean body weights at the start of the experiment. All the rats gained weight throughout the six-week study. Starting from week three until the completion of the study, the ovariectomized rats showed significantly higher body weights compared with the other rats. Tocotrienol supplementation inhibited the increase in body weight observed following ovariectomy (Figure 1). The ovariectomized rats had a higher average daily food intake compared with the sham and tocotrienol-treated groups. The daily food intake of rats supplemented with tocotrienol did not differ significantly from that of the sham rats (Table 1).
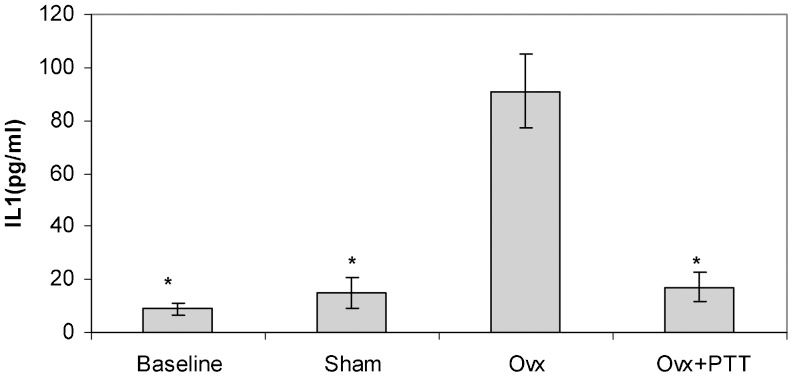
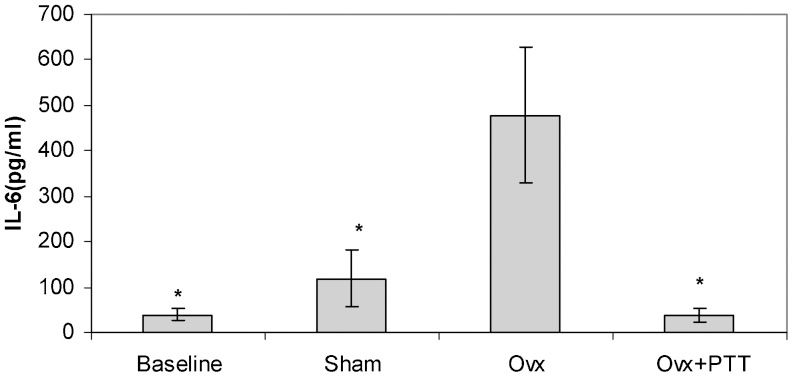
As shown in Figures 2 and 3, ovariectomy caused an increase in the levels of both interleukin-1 and interleukin-6. Treatment with tocotrienol successfully prevented this rise in cytokine levels.
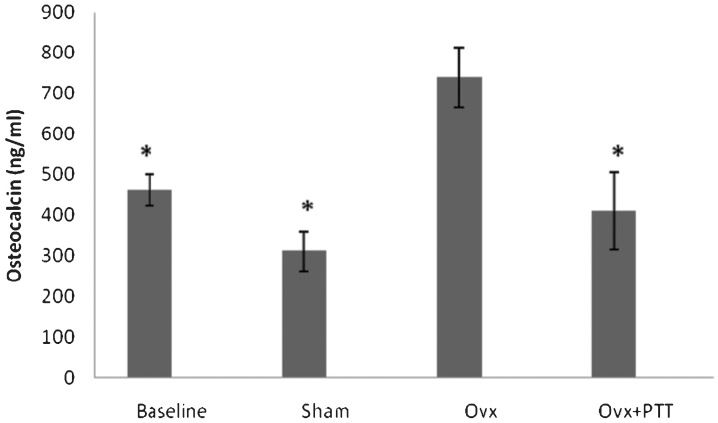
The osteocalcin level in tocotrienol-treated rats was significantly reduced compared with the ovariectomy control group (Figure 4).
Serum pyridinolineNo significant differences were noted in the levels of serum pyridinoline in any of the groups of rats (data not shown).
DISCUSSIONThe purpose of this study was to evaluate the effects of pure tocotrienol on bone metabolism in ovariectomized female rats by measuring biochemical parameters including the levels of bone-resorbing cytokines (interleukin-1 and interleukin-6), a bone formation marker (osteocalcin) and a bone resorption marker (serum pyridinoline). The ovariectomized rat has become a widely accepted model of human postmenopausal osteoporosis because of the many similarities in their pathophysiological mechanisms (11,12). In both species, bone loss occurs rapidly after the onset of estrogen deficiency, resulting in a reduction in bone mineral density (13). The responses to mechanical influences (e.g., exercise) and various treatment modalities are also similar (14). Although rats are skeletally mature at 10 months old, the cheaper and more readily available three-month-old rats are generally used because their bone loss characteristics are similar to those of the aged rat model. Rats younger than 3 months old are not suitable models for studying bone loss caused by ovarian deficiency because the normal processes of bone remodeling and skeletal maturation during growth may mask the effects of ovariectomy-induced bone loss (15).
In our previous study, we observed no reduction in trabecular bone volume in ovariectomized rats supplemented with pure palm tocotrienol. All the structural parameters (with the exception of trabecular thickness) were maintained at the levels of sham rats (16). The results of the structural histomorphometric analysis suggest that tocotrienols prevent bone resorption by reducing the trabecular perforation that typically occurs during estrogen deficiency. We also reported that palm tocotrienols significantly improved bone dynamic parameters in estrogen-deficient rats by increasing the mineralizing surface, the mineral apposition rate and the bone formation rate (17). The effects of tocotrienol on dynamic parameters indicate that this form of vitamin E has anabolic properties that result in increased bone formation. This observation has been confirmed in a study performed by Shuid et al. (18).
Ovarian hormone deficiency increases bone resorption, which results in bone mineral loss from the skeleton. Lack of estrogen disrupts calcium homeostasis, leading to a negative calcium balance via a combination of decreased intestinal absorption and increased renal excretion (19). In addition, reduced estrogen contributes to oxidative stress by producing excessive free radicals. This process is associated with low levels of antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase and glutathione-S-transferase (20). These enzymes are responsible for metabolizing free radical molecules to non-radical products. Osteoporotic patients were found to have low levels of antioxidants (21) and high levels of free radical species (22). Although toxic to the bone-forming osteoblasts (23), free radicals activate osteoclastic bone resorption, which is responsible for bone loss (5). Bone resorption in postmenopausal osteoporosis has also been linked to the inflammatory process. Various studies have demonstrated the connection between osteoclasts, M-CSF and inflammatory cytokines such as TNF-α and interleukins 1, 6 and 7. Estrogen deficiency causes increased production of inflammatory cytokines, which stimulate osteoclasts to resorb bone via the binding of RANKL to its receptor (RANK) (24).
In our current study, ovariectomized rats had elevated levels of interleukins 1 and 6. The osteocalcin level, which is a marker of bone formation, was also increased. This is consistent with previous findings that bone loss in ovariectomy is due to a high bone turnover rate with resorption exceeding formation (11,25). The increase in the levels of cytokines and osteocalcin in ovariectomized rats was prevented by supplementation with tocotrienols. This finding is in agreement with our earlier studies, in which tocotrienols were able to reduce the interleukin-1 and interleukin-6 levels in a free radical-induced osteoporotic rat model (9).
The lack of a serum pyridinoline response in this study could be due to the short duration of the study period. Most studies examining serum pyridinoline were carried out for longer durations, the average being 16 weeks (26,27). In these studies, serum pyridinolines in ovariectomized animals were higher than those in the sham group. Equivalent results were obtained in studies using urinary pyridinoline as a marker for bone resorption (28).
Our histomorphometric and biochemical results strongly indicate that palm tocotrienol was able to prevent the loss of trabecular bone in accelerated bone loss conditions such as osteoporosis. An earlier study using palm vitamin E (which contains a higher percentage of tocotrienols than tocopherols) showed that this vitamin was effective in preventing the loss of bone mineral density in osteoporotic male rats (29). The same study also showed that palm vitamin E increased the calcium content in rats with osteoporosis induced by orchidectomy. Similarly, a study performed by Norazlina et al. (30) showed that vitamin E deficiency impaired bone calcification, whereas supplementation with palm vitamin E improved the bone calcium content in growing rats.
The role of antioxidants in scavenging reactive oxygen species during oxidative stress is well established. Tocotrienol is a powerful antioxidant from the vitamin E family. This family consists of eight naturally occurring isoforms including α-, β-, γ- and δ-tocopherols as well as α-, β-, γ- and δ-tocotrienols. Significant attention has been focused on tocopherol, most likely because of its abundance. Tocotrienols are similar to tocopherols except that they possess three trans double bonds in the hydrocarbon tail instead of a saturated phytyl tail (31). This unique structure makes tocotrienol a more potent antioxidant compared with tocopherol.
Recent scientific studies have shown that tocotrienol is better able to scavenge free radicals compared with tocopherol (32–34), most likely because the unsaturated side chain of tocotrienol penetrates tissues more efficiently, allowing it to be better distributed in the fatty layers of the cell membrane (35). Other beneficial effects of tocotrienols such as their anti-cancer, neuroprotective and cholesterol-lowering properties, have also been reported (36–38). We did not measure free radicals or antioxidant status in this experiment, although other studies have shown that ovariectomy caused an increase in reactive oxygen species and a decrease in the levels of thiol antioxidants, whereas administration of 17-beta-estradiol reversed these effects and prevented bone loss (6,39). We did, however, conduct a study to measure the level of thiobarbituric acid-reactive substances (TBARSs), which is an index of lipid peroxidation, and the levels of the antioxidant enzymes glutathione peroxidase and superoxide dismutase in the femurs of normal male rats (40). It was shown that rats supplemented with tocotrienol had a lower TBARS level; however, the glutathione peroxidase activity was increased. This finding indicates that tocotrienol directly enhances the antioxidant defense system in bone. Another study also showed that supplementation with tocotrienol more successfully prevented bone loss in rats exposed to nicotine compared with tocopherol (41). Therefore, modern treatments for osteoporosis should be directed toward increasing the bone antioxidant defense, and tocotrienol should be considered in the choice of therapy.
In conclusion, treatment with tocotrienol at a dose of 60 mg/kg body weight prevented bone loss due to estrogen deficiency. Tocotrienol can be potentially used to prevent postmenopausal osteoporosis in women, provided that sufficient clinical studies are carried out to establish the efficacy and safety of this form of antioxidant vitamin.
AUTHOR CONTRIBUTIONSMuhammad N prepared the manuscript and was involved in performing the study and in data collection, analysis and interpretation. Soelaiman IN designed the study and supervised all aspects of the project. Luke DA provided conceptual advice, particularly for bone histology. Shuid AN, Mohamed N and Soelaiman IN assisted in the design of the study. All authors contributed to the interpretation of the results and the determination of the study implications. Additionally, all authors have reviewed the manuscript.
The authors would like to thank the Universiti Kebangsaan Malaysia for funding this project under a faculty grant. We would also like to thank Ms. Shahani Muhamad, Mr. Faisal Ariffin and Mr. Mohamad Arizi Aziz for their technical assistance. We would like to express our gratitude to Dr. Haizal M. Hussaini for allowing us to use the image analyzer, and we thank Mr. Abdul Gapor M. Top from the Malaysian Palm Oil Board for supplying the tocotrienol used in this study.
No potential conflict of interest was reported.