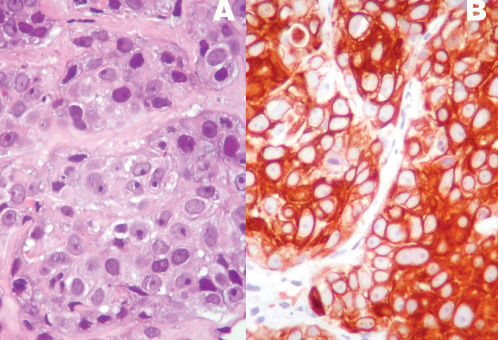
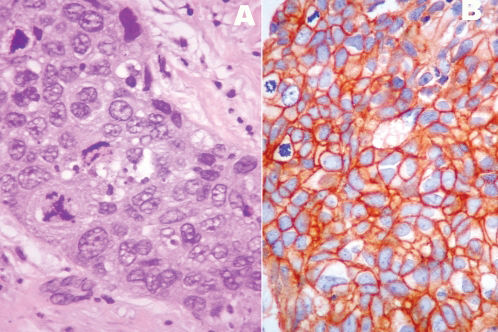
To compare the frequency and immunohistochemical profiles of triple-negative breast carcinomas in younger and older women.
METHODS AND RESULTS:We selected patients diagnosed with triple-negative breast carcinomas. The groups examined were women who were 35 years old or younger between 1997 and 2007 (n = 74) and, for comparison, women who were 60 years old or older (n = 19, consecutive cases). All formalin-fixed and paraffin-embedded tumor samples were reviewed and immunohistochemically stained for ER, PR, HER2, Ki-67 antigen, epidermal growth factor receptor, cytokeratin 5/6, p53, vimentin, CD117, and p63 using tissue microarrays blocks. Triple-negative breast carcinomas corresponded to 34.6% (74/213) of the carcinomas from the younger patients and 16.2% (19/117) of the carcinomas from the older patients (p = 0.002). No significant differences in the frequency of the basal phenotype were observed in the two patient groups based on CK5/6 and/or epidermal growth factor receptor expression (74.3% vs. 68.4%). However, triple-negative breast carcinomas in the older patients presented a higher frequency of CK5/6 expression compared to those of younger patients (42.1% vs. 9.6%; p = 0.005), whereas triple-negative breast carcinomas of younger patients had a higher expression level of epidermal growth factor receptor (71.6% vs. 47.3%).
CONCLUSIONS:These results show that there were significant molecular differences between the triple-negative basal-like breast carcinomas that were diagnosed in younger women and those that were diagnosed in older women. These findings may provide a basis for describing the more aggressive phenotype of the triple-negative breast carcinomas observed in younger women.
Breast cancer detected in younger patients has been associated with a more aggressive phenotype.1-3 To improve treatment outcomes and to reduce mortality from this disease, a greater understanding of this aggressive phenotype is needed. Gene-expression profiling using DNA microarrays has identified five subtypes of breast cancer (i.e., luminal A, luminal B, normal breast-like, HER2-overexpression, and basal-like). Each of these subtypes is associated with a distinct prognosis, but the different subtypes share similarities. For example, immunohistochemistry has been used to evaluate the expression of estrogen receptors (ERs), progesterone receptors (PRs), HER2, and Ki-67 to characterize the different subtypes.4 The basal-like and HER2+ subtypes both have a shorter relapse-free survival and overall survival than luminal tumors.5,6 Triple-negative (TN) breast carcinomas (i.e., ER-negative, PR-negative, and HER2-negative tumors) have been shown to be related to a basal-like phenotype and, accordingly, exhibit more aggressive clinical and pathologic features.7 Triple-negative carcinomas are also more prevalent among specific subgroups of women, particularly younger patients, and have been found to be associated with BRCA1 germline mutations.8-11 Although significant overlap in criteria of classification of TN, basal-like carcinomas, and BRCA1-related tumors has been shown, these three tumor types are not synonymous. Therefore, no consensus exists regarding the criteria that identify basal-like subgroups of TN carcinomas. Currently, the most useful criteria have been the expression of basal-cytokeratin (CK5/6) and/or epidermal growth factor receptor (EGFR);12 however, recent studies have indicated that TN carcinomas are not as homogeneous as they were first thought to be.7,13,14 In this study, the frequencies and phenotypic characteristics of TN and basal-like carcinomas in patients who were 35 years old or younger were analyzed and compared with those of TN carcinomas diagnosed in patients who were 60 years old and older.
METHODS AND MATERIALSThis project was approved by the Scientific Committee of the Department of Pathology of the Faculdade de Medicina da Universidade de Sao Paulo and by the Ethical Committee for Research Projects of the Hospital das Clinicas da Faculdade de Medicina da Universidade de Sao Paulo (CAPPesq) (protocol 563/07). Between 1997 and 2007, 213 tumor specimens from patients who were 35 years old or younger were registered at the Consultoria em Patologia, a large reference laboratory located in Botucatu, Sao Paulo, Brazil. An additional 117 tumor samples from patients who were 60 years old or older were also analyzed by the same laboratory in 2006. All tumor samples from the two age groups were reviewed and classified according to histological type, histological grade, nuclear grade, the presence of tumor necrosis, the presence of an in situ component, and any vascular involvement.
For each tumor, representative areas were selected to construct tissue microarrays (TMAs). Briefly, three cylinders, each 1.5 mm in diameter, were removed from selected areas of donor blocks and mounted into paraffin blocks at 1-mm intervals using a precision microarray instrument (Beecher Instruments, Silver Spring, MD). A grid system was established, and each core had a coordinate reference (i.e., x-axis, y-axis) for sample identification. Blocks were sealed at 60°C for 10 min, and 5-µm sections of the resulting TMA blocks were prepared using standard techniques and mounted on Starfrost® slides.
Histological sections from the TMA blocks were immunostained for ER, PR, HER2, Ki-67 antigen, EGFR, CK5/6, p53, vimentin, CD117, and p63. The sources and dilutions of the antibodies and the epitope retrieval methods that were used are listed in Table 1. Bound antibodies were detected using Novolink system (Leica, USA).
Reagents and methods used for immunohistochemical analysis.
| Antigen | Clone/Source | Dilution | Epitope retrieval method |
|---|---|---|---|
| ER | R; SP1/Thermo Scientific | 1 ∶ 500 | Pressure cooker, 9 min |
| PR | M; PgR636/Dako | 1 ∶ 1000 | Pressure cooker, 9 min |
| HER2 | R; SP3/Thermo Scientific | 1 ∶ 100 | Microwave oven |
| p53 protein | M; DO-7/Dako | 1 ∶ 2700 | Pressure cooker, 8 min |
| Ki-67 | M; MIB1/Dako | 1 ∶ 600 | Pressure cooker, 8 min |
| EGFR | M; 31G7/Zymed | 1 ∶ 200 | 0.1% Pronase, RT, 15 min |
| p63 protein | M; 4A4/Dako | 1 ∶ 300 | Pressure cooker, 8 min |
| Vimentin | M; V9/Dako | 1 ∶ 200 | Microwave oven |
| c-kit (CD117) | R; polyclonal/Dako | 1 ∶ 50 | Microwave oven |
| CK5/6 | M; D5/16B4/Dako | 1 ∶ 100 | Microwave oven |
ER: Estrogen receptor; PR: Progesterone receptor; EGFR: Epidermal growth factor receptor; CK: cytokeratin
Pressure cooker: citrate buffer (pH 6) (Tender Cooker, Nordic Wave, USA)
Microwave: citrate buffer (pH 6), 15 min (Eletrolux, 900 W)
Positive immunohistochemistry staining was used to identify cytoplasmic localization of CK5/6, vimentin, and CD117; nuclear localization of ER, PR, Ki-67, p63, and p53; and membranous-pattern staining of EGFR and HER2. In this study, only cases that had no ER- or PR-positive cells, were negative for HER2, and were scored as 0 or 1+ according to the guidelines of the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP)15 were included. Ki-67 expression was scored as either < 25% or > 25%. For the other markers, at least 1% of the positive cells with a moderate to strong intensity were considered positive.
Statistical analysis was performed using a two-way contingency table and the chi-square test.
RESULTSTriple-negative breast carcinomas were diagnosed in 74/213 (34.7%) patients who were 35 years old or younger and in 19/117 (16.2%) patients who were 60 years old or older (p = 0.002). The median ages of the younger and older patients who were included in the study were 32 years (mean ± SD: 30.9 ± 3.94; range: 21-35 years) and 73 years (mean ± SD: 73.7 ± 7.53; range: 61-91 years), respectively. In the younger group, 67/74 (90.5%) tumors were of a ductal histological type; other histological types identified included metaplastic (2 cases), pleomorphic lobular (2 cases), secretory (1 case), mucinous (1 case), and medullary (1 case). Regarding the older patients, 15/19 (78.9%) tumors were of a ductal histological type, and 4 (21.1%) were of a pleomorphic lobular type. The pathological features of the tumors that were identified in each patient group are summarized in Table 2. The histological and nuclear grades and the angiovascular involvement were not different between the two patient groups. Tumoral necrosis was more frequently observed in tumors that were derived from younger patients than in those from older patients (52.7% vs. 31.6%, respectively). However, the difference was not statistically significant. The basal phenotype, which was determined by the expression of EGFR and/or CK5/6 according to the criteria of Nielsen et al.,12 was similar for both groups (74.3% for younger patients vs. 68.4% for older patients). However, the two markers showed differences in the frequency between the two groups (Figures 1 and 2). Tumors that were derived from the younger patients were more frequently EGFR positive (71.6% vs. 47.3%, respectively) and exhibited a lower frequency of CK5/6 expression (9.4% vs. 42.1%, respectively). Regarding the basal-like tumors of younger patients, 53/55 (96.4%) were associated with EGFR expression, whereas in the older patient group, 9/13 (69.2%) expressed EGFR (p = 0.0018). c-KIT expression was also observed more frequently in tumors that occurred in older patients (36.8%) than in younger patients (21.2%), although the difference was not significant. The analysis of other markers, including Ki-67, p63, p53, and vimentin, did not show any significant differences between the two groups.
Pathological and immunohistochemical features of TN Breast carcinomas in younger (≤ 35 years) vs. older (≥ 60 years) patients.
| Variable | ≤ 35 years (n = 213) N (%) | ≥ 60 years (n = 117) N (%) | p-value1 |
|---|---|---|---|
| TN carcinomas | 74 (34.7) | 19 (16.2) | 0.0004 |
| Histological grade 3 | 56 (75.7) | 14 (73.7) | NS2 |
| Nuclear grade 3 | 55 (74.3) | 14 (73.7) | NS |
| LVI3 | 12 (16.2) | 2 (10.5) | NS |
| Tumoral necrosis | 39 (52.7) | 6 (31.6) | NS |
| Basal-like molecular profile4 | 55 (74.3) | 13 (68.4) | NS |
| CK5/6 | 7 (9.4) | 8 (42.1) | 0.0005 |
| EGFR | 53 (71.6) | 9 (47.3) | 0.0045 |
| Ki-67 (>25%) | 51 (68.9) | 12 (63.1) | NS |
| p63 | 1 (1.3) | 0 | NS |
| p53 | 26 (35.1) | 7 (36.8) | NS |
| Vimentin | 31/71 (43.7)5 | 5/18 (27.8)5 | NS |
| c-kit | 15/71 (21.2)5 | 7 (36.8) | NS |
Breast cancer that is detected in younger women is typically associated with aggressive behavior and results in a poor prognosis. Unfortunately, the specific mechanisms responsible for this tumor phenotype remain unclear. This poor understanding is further complicated by the controversial results of many studies. Currently, breast cancers that are diagnosed in younger patients are characterized by reduced hormone sensitivity and high HER2/EGFR expression,2,16 although ER-positive carcinomas are still a prevalent subgroup of tumors in this age group.1,16 Furthermore, luminal carcinomas in younger women are mostly subtype B, which is determined by their frequent co-expression of HER2 and/or their high proliferative activity.16 Triple-negative carcinomas tend to occur less frequently than luminal carcinomas; however, triple-negative carcinomas are more prevalent in patients who are younger than 35 years old.17
TN carcinomas are considered to be a group of biologically distinct neoplasias that mostly exhibit a basal phenotype and an aggressive biology. Unlike the other subtypes, targeted agents that are specifically aimed at triple-negative breast tumors are not yet available; thus, this deficiency intensifies the need and interest in advancing novel therapeutic strategies beyond chemotherapy for this subset of high-risk patients. Triple-negative is a term based on clinical assays for ER, PR, and HER2, whereas basal-like is a molecular phenotype that was initially defined using cDNA microarrays. However, low-grade tumors, such as adenoid cystic and medullary carcinomas, are also considered TN carcinomas.13,18 Therefore, the best criteria to characterize the phenotype of basal carcinomas have remained somewhat controversial. Nielsen et al. proposed that the best immunohistochemistry predictors of basal-like gene expression are the positive expression of CK 5/6 and EGFR and the negative expression of HER2 and ER; these criteria were associated with a sensitivity of 76% and a specificity 100%.12 In this study, we analyzed these markers; however, it should be noted that other studies have considered the expression patterns of CK5, CK14, CK 17, p-cadherin, p63, c-kit, and vimentin to characterize a basal-like phenotype.19-22 Differences in the molecular profile of basal-like carcinomas have also been associated with protein expression patterns of physiological stem/progenitor cells during breast development.23 In this histogenetic model, adult CK5-positive stem cells are hypothesized to differentiate into neoplastic stem cells as a result of EGFR amplification, a mutation of p53, or BRCA inactivation, and this differentiation leads to the generation of basal-like carcinomas. In general, these types of tumors express high levels of p-cadherin, EGFR, and CK5 and an absence or low levels of luminal markers, such as CK 8/18.23 Given the number of markers involved, the possibility that different expression profiles could define distinct subsets of basal-like carcinomas should be considered. For example, Rakha et al. compared tumors with a basal phenotype that were characterized by the expression of basal cytokeratins (CK5/6 or CK14) with cells with a myoepithelial phenotype (p63 or smooth muscle actin).24 The basal phenotype was associated with a worse outcome, which suggested that although basal and myoepithelial phenotypes share many features, including a similar genetic profile, they possible are distinct tumors.24
Very few studies exist that have examined molecular differences in breast tumors according to age.1-3,9,16,25 In the present study, a higher prevalence of TN carcinoma was observed in younger patients (34.3% for younger patients vs. 16.3% for older patients), and a basal-like phenotype associated with positive expression of CK5/6 and/or EGFR was present in 74.3% vs. 68.4%, respectively. More importantly, the molecular profiles of the TN carcinomas from younger patients were different from the profiles of the tumors from older patients. Triple negative carcinomas in the younger age group were more frequently EGFR positive (71.6% vs. 47.3%, respectively) and less frequently CK5/6 positive (9.4% vs. 42.1%, respectively). The role of EGFR expression in breast cancer has been investigated by many authors.11,14,26,27 Viale et al. reported a worse prognosis for patients with TN carcinomas that had EGFR-immunoreactivity present in more than 50% of neoplastic cells, which suggested a possible prognostic role for quantification.14 Similarly, Rimawi et al. used radioligand-binding assays to quantify EGFR expression in frozen sections of breast tissue carcinomas and found that higher levels of EGFR were associated with younger and black women, a more aggressive outcome, and lower levels of hormone receptors.27 The relationship between EGFR and BRCA1 has been investigated by different groups, and BRCA1-germline mutations have been associated with a basal-like phenotype and younger age.8-11 In a study by Collins et al. that analyzed BRCA1 in 144 TN carcinomas, independent of the patient's age,26 they found a higher prevalence of basal CK and EGFR among TN breast cancers; however, the frequency of expression of these markers was similar in women with and without BRCA1 mutations.26 In contrast, Arnes et al. found that EGFR was a predictor of BRCA1 status, followed by patient age and ER status.11 In the same study, a worse prognosis was associated with a mutation of BRCA1 and positive expression of EGFR.11
As a receptor tyrosine kinase that plays essential roles in both normal physiological conditions and cancerous conditions, EGFR can affect many important characteristics of a cancer's phenotype, including evasion of apoptosis, proliferation, invasion, and metastasis. In a previous study, the frequency of EGFR-positive tumors among young patients was found to be higher than among older women (5.9% vs. 3.3%, respectively), although the difference was not statistically significant.16 Based on the results of that study and the data presented in this report, EGFR expression appears to play an important role in the early onset of breast cancer, and we hypothesize that this role is related to intrinsic genetic differences that result in an adverse outcome that is associated with the aggressive breast carcinomas. Furthermore, we propose that EGFR immunostaining and/or amplification should be further investigated as predictor of a patient's response to targeted therapy.
This work was supported by FAPESP (Fundação de Amparo a Pesquisa do Estado de Sao Paulo): grant #2007-03139-9 (FMC) and scientific initiation scholarship #07/51613-1 (LMB).