The promotion of extracellular matrix synthesis by chondrocytes is a requisite part of an effective cartilage tissue engineering strategy. The aim of this in vitro study was to determine the effect of bi-axial cyclic mechanical loading on cell proliferation and the synthesis of glycosaminoglycans by chondrocytes in three-dimensional cultures.
METHOD:A strain comprising 10% direct compression and 1% compressive shear was applied to bovine chondrocytes seeded in an agarose gel during two 12-hour conditioning periods separated by a 12-hour resting period.
RESULTS:The bi-axial-loaded chondrocytes demonstrated a significant increase in glycosaminoglycan synthesis compared with samples exposed to uni-axial or no loading over the same period (p<0.05). The use of a free-swelling recovery period prior to the loading regime resulted in additional glycosaminoglycan production and a significant increase in DNA content (p<0.05), indicating cell proliferation.
CONCLUSIONS:These results demonstrate that the use of a bi-axial loading regime results in increased matrix production compared with uni-axial loading.
Mechanical loading is an essential factor in the maintenance of articular cartilage matrix homeostasis (1) and is linked to both matrix composition (2) and remodeling (3,4). This interaction has been determined in both in vitro and in vivo studies, which have both demonstrated that mechanical loading effectively modulates chondrocyte metabolism (5-10). Through a complex and poorly understood mechanotransduction pathway, chondrocytes respond to a variety of loading regimes in different ways: static compression or the lack of loading has been shown to cause matrix degradation, but some forms of dynamic compression have been shown to maintain matrix homeostasis or foster an increase in matrix density (5,6,11). Physiological loading patterns produced by gait and walking activities comprise a combination of loading forms, varying in terms approximately equivalent to frequency, force, waveform and duration and including a variable mix of static-, compressive-, and shear- loading components (12). While previous studies have examined the effect of static compression, directly or indirectly applied pressure (including hydrostatic pressure, osmotic pressure, pH and ion concentration and interstitial fluid flow–induced pressure), compressive stresses, and sliding shear stress on chondrocyte metabolism, there have been no studies published to date that have examined the effect of a bi-axial loading regime comprised of the cyclic application of both compressive and shear loading on isolated chondrocytes, which would ensure the full recovery of dimension within each cycle.
Given that existing research indicates that chondrocytes are able to differentiate different loading regimes in terms of a differential response and given the complex nature of cartilage tissue, which makes consistent loading of chondrocytes difficult, the present study makes use of the well-established chondrocyte-seeded agarose model and a bioreactor capable of highly flexible combinations of compressive and shear loading over a sustained period.
This study aims to test the hypothesis that bi-axial loading increases sulfate glycosaminoglycan synthesis, a key component of the extracellular matrix compared with compressive loading alone. In addition, based on previous reports suggesting that a post-isolation recovery period is beneficial, the effect of a period of free-swelling recovery prior to the mechanotransduction by the chondrocytes is evaluated and quantified (5,13).
MATERIALS AND METHODSBiochemical reagentsAll reagents, buffers and assays were obtained from Sigma-Aldrich (Petaling Jaya, Selangor, Malaysia) unless otherwise stated. The culture medium was Dulbecco's Modified Eagle's Medium (DMEM) (D5921) supplemented with 20% fetal bovine serum (FBS), 2% (v/v) HEPES buffer solution, 1% (v/v) penicillin-streptomycin, 1% (v/v) L-glutamine and 0.01% (w/v) L-ascorbic acid. The culture medium was sterilized using a 0.22 μm cellulose acetate filter.
Preparation of chondrocyte/agarose constructsBovine articular cartilage was obtained from cow joints from the local abattoir. The cells were isolated on the same day the 18–24 month old Bos indicus calf was slaughtered. Articular chondrocytes were isolated from dissected bovine metacarpal-phalangeal joints. The full thickness of the cartilage from the entire proximal surface of the joint was removed under sterile conditions. The explanted tissue was then enzymatically digested with 20 U·ml-1 protease at 37°C for 1 hour and immersed in 200 U·ml-1 collagenase type II for a further 16 hours. In both cases, the enzymes were resuspended in DMEM supplemented with 20% FBS. To ensure that all cartilage fragments were fully exposed during digestion, the Falcon tubes containing the cartilage fragments were placed on a roller-mixer (ProBlot L12-2, LabNet, Malaysia).
The supernatant containing the released chondrocytes was passed through a 70 μm cell sieve (BD Bioscience, Malaysia) into sterile Falcon tubes and washed twice with DMEM+20% FBS. Cell suspensions from several joints were pooled, resuspended to a uniform density of live cells, and mixed with an equal volume of 8% agarose (type VII) prepared in Earl's Balanced Salt Solution (EBSS) to yield a 4% w/v agarose gel containing 4×106 cells·ml-1. The cell/agarose suspension was transferred while still fluid into a mold to form six 5 mm cubes, which were then affixed on two opposite faces to strips of sintered glass that were used in the subsequent mechanical manipulations.
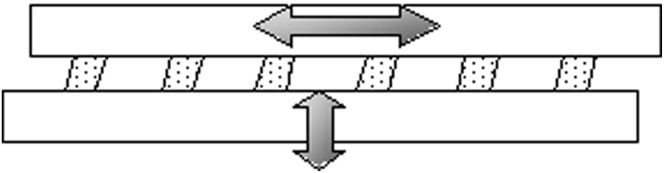
Bi-axial Loading SystemAn incubator-housed bioreactor (14) was used to facilitate the direct compression and shear compression applied to the chondrocyte/agarose constructs. The bioreactor uses strips of sintered glass to which the cell/agarose constructs are affixed to permit the long-term application of cyclic displacement in both the principal compressive and shear axes (Figure 1). For uni-axial loading, a 10% compressive strain was delivered to the constructs; for bi-axial loading, the 10% compressive strain was combined with a 1% shear strain. The loading frequency was maintained at 1 Hz, and the waveform was sinusoidal throughout all experiments. The system was operated according to the method of Yusoff et al. (14).
Schematic diagram showing six agarose constructs seeded with chondrocytes attached to porous glass strips. The top strip moves vertically to give a shearing effect, while the other strip moves horizontally for the compressive strain. The movement of the porous glass strips is controlled by the bioreactor.
The created constructs were divided into three equal groups: one group was subjected to uni-axial loading, another to bi-axial loading, and the third left unloaded for an equivalent period. The loading took place for two 12-hour periods, separated by a 12-hour recovery time. An additional comparative subdivision across all groups added a free-swelling recovery period of 24 hours post-isolation and seeding before the loading was initiated. Each group contained 24 constructs, and the data were obtained across four time-separated repeats. The groups, divisions, loading regimes and repeats per group are summarized in Table 1.
Outline of the experiments performed to assess the influence of bi-axial loading and uni-axial loading and the need for pre-culturing prior to the chondrocytes being subjected to the mechanical loads. Uni-axial samples were exposed to 10% direct compressive strain and 0% shear strain, whereas bi-axial samples were exposed to 10% direct compressive strain and 1% shear strain. In the experimental setups, (○) signifies chondrocytes that were subjected to loads immediately upon cell seeding, and (•) signifies chondrocytes that were left pre-cultured for 24 hours prior to being subjected to any load.
| Group and sub-group | ||||||
|---|---|---|---|---|---|---|
| Loading | Uni-axial loading | Bi-axial loading | No loading | |||
| Free-swelling | ○ | • | ○ | • | ○ | • |
| Loading Pattern | ||||||
| -12–0 h: free swelling | n/a | Rest | n/a | Rest | n/a | Rest |
| 0–12 h | Uni-axial | Uni-axial | Bi-axial | Bi-axial | Rest | Rest |
| 12–24 h | Rest | Rest | Rest | Rest | Rest | Rest |
| 24–36 h | Uni-axial | Uni-axial | Bi-axial | Bi-axial | Rest | Rest |
| 36–48 h | Rest | Rest | Rest | Rest | Rest | Rest |
| Repeats and samples per batch | ||||||
| n (per batch) | 6 | 6 | 6 | 6 | 6 | 6 |
| n (total) | 24 | 24 | 24 | 24 | 24 | 24 |
| Total constructs | 144 | |||||
If a batch was discovered to be infected, then it was immediately destroyed, and no results from that batch were included in the study; this situation occurred in two cases. Furthermore, if detachment of the constructs occurred during the course of the experiment, the data were omitted from analysis as it was no longer possible to determine how much load the constructs had been subjected to. This occurred once, and the experiment was repeated.
Biochemical AnalysisFollowing mechanical stimulation, all constructs were enzymatically digested in 10 U·ml-1 of agarase and 2.8 U·ml-1 of papain (15). The suspension was analyzed for DNA content using Hoechst 33258 dye with calf thymus DNA as a standard (16). A microplate-based fluorometer (FLUOstar Optima, BMG Labtech, Ofdenberg, Germany) was used to obtain the fluorescence levels (450 nm emission, 355 nm excitation) (16,17). The glycosaminoglycan (GAG) content in both the medium and agarase/papain digests was assessed using a DMB assay with a chondroitin-4-sulfate standard (18). As collagen is not expected to be synthesized in significant quantities during the time span of this experiment, it was not measured.
Statistical AnalysisAll data are presented as the mean normalized to the values obtained from the unstrained samples with the standard error of the mean values across the 24 replicates from 4 separate experiments used in each group. ANOVA was used to determine the significance of the differences between the groups tested. All statistical analyses were performed using SPSS (Version 17.0; SPSS Inc., Chicago, Illinois, USA, 2006), with the significance level set at α = 0.05. A post hoc LSD test was used for multiple comparisons at α = 0.05.
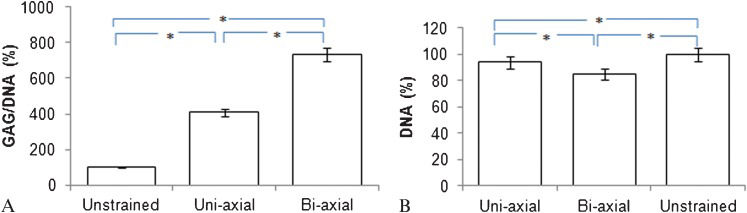
RESULTSEffects of bi-axial and uni-axial loading on GAG synthesis and DNA contentThe levels of GAG synthesis observed from cells exposed either to 10% uni-axial compression or the same compression combined with 1% shear compressive strain are shown in Figure 2A. The presented data have been normalized to the unstrained control samples to limit the effect of batch-to-batch variation and any changes in environmental conditions. A total of 24 samples were used for each condition, with sets of six samples tested within each batch; the batches were separated by one week, and fresh cells were obtained from new calves for each batch. There was an approximately 50% increase in GAG production after the addition of the 1% shear loading cycle.
After a free-swelling pre-culture for 24 hours, the chondrocyte/agarose constructs were subjected to either 10% direct compressive and 1% shear compressive strain (Bi-axial) or 10% direct compressive strain without shear strain (Uni-axial). At the end of the experiment, (a) proteoglycan synthesis and (b) DNA content were quantified. The values are depicted as the percentage change and standard error. The values are normalized to unstrained samples as the control group (100%). The DNA content of each sample is treated as the baseline in (a). As determined using ANOVA, all data from the samples loaded bi-axially or uni-axially were significantly different. (∗) p<0.05.
The DNA content of the samples that were exposed to sinusoidal loading was reduced approximately 10% when compared with the unstrained samples (p<0.05) (Figure 2B. However, even after normalization to the DNA content, GAG production in the bi-axially loaded constructs was 1.8-fold higher than the production in the uni-axial loaded constructs (p<0.05) (Figure 2A).
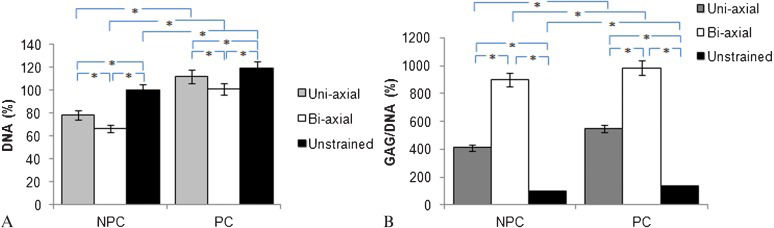
Effects of pre-culturing on GAG synthesis and DNA contentFigure 3 summarizes the results obtained in cells that had been pre-cultured or cells that were not pre-cultured. The pre-cultured (PC) samples demonstrated higher cellularity and GAG production than the non-pre-cultured (NPC) samples. There was a 30% increase in the DNA content of the PC samples when the constructs were subjected to biaxial loading (Figure 3A). Additionally, a statistically significant 10% increase in GAG production was observed in the PC samples (Figure 3B) (p<0.05).
The (a) DNA content of and (b) proteoglycan production by chondrocytes seeded in agarose subjected to bi-axial (10% compressive + 1% shear strains) or uni-axial (10% compressive + 0% shear strains) loads immediately after cell seeding or after 24 hours of pre-culture under free-swelling conditions was measured. The data were taken from a total of 48 samples for each pre-cultured (PC) and non-pre-cultured (NPC) condition. The data are presented as the percentage change normalized to the non-pre-cultured samples. The DNA content of the chondrocytes in each sample is the baseline for all glycosaminoglycan measurements for all conditions in (b). Unstrained non-pre-cultured samples are treated as the control group. The error bar depicts the standard error of the mean. (∗) p<0.05.
Worldwide, one in ten people has osteoarthritis, which is a recurring progressive degenerative disease that is usually caused by trauma or overuse of the afflicted joint (19). Although some tissues might be expected to undergo repair when given appropriate support and rest, repair of the articular cartilage is rarely observed. Two important factors of a tissue engineering solution for articular cartilage are the extent to which a functional matrix can be produced and the ability to stimulate and sustain cell proliferation. The role of mechanical load in cartilage tissue remodeling and chondrocyte signaling has been clearly established (20-22).
Previous studies have shown that low amplitude dynamic compression induces the stimulation of GAG synthesis in cartilage explants (23-26) and isolated chondrocytes cultured in three-dimensional structures (5-9,15). Physiologically, articular cartilage in the load-bearing joints experiences complex mechanical loading consisting of combinations of compressive, shearing and tensile forces (27). Most studies have used animal models as primary animal material is more easily acquired, although a few studies have demonstrated comparable trends with human cartilage. Table 2 summarizes the results of some studies using human and bovine cartilage that have examined the effect of physiological mechanical loading on matrix synthesis by chondrocytes.
Comparison of previous in vitro studies indicating the positive effect of mechanical loading on articular chondrocytes.
| Type of load | Regimen | Model System | Major Effect | Reference |
|---|---|---|---|---|
| Dynamic compression | 15% amplitude strains at a frequency of 0.3 Hz | Bovine articular chondrocytes | After 48 hours, an increase in proteoglycans and collagens was observed | (9) |
| Dynamic compressive and shear loading | 2–5% strain at a frequency of 0.5 Hz | Bovine articular chondrocytes | Increased proteoglycan and collagen levels | (10) |
| Intermittent hydrostatic pressure | 10 MPa at a frequency of 1 Hz | Human osteoarthritis chondrocytes | Increased Type II collagen and SOX9 gene expression | (28) |
| Intermittent hydrostatic pressure | 1, 5 and 10 MPa | Human articular chondrocytes | Upregulated Aggrecan and type II collagen mRNA | (29) |
| Cyclic pressure–induced strain | 0.33 Hz | Human articular chondrocytes | Increased Aggrecan mRNA | (30) |
In the present study, the differential effect of uni- and bi-axial loading on the chondrocytes demonstrates that bi-axial loading is advantageous for the synthesis of GAG, resulting in an approximately 50% increase. Our findings are consistent with the results reported following the application of 1–3% shear strain alone (24,31).
Some of the possible mechanisms involved in the transduction of dynamic compression include altered fluid pressure, enhanced fluid flow, induced streaming potentials, cell-matrix interactions and growth factor release. In addition to cellular and nuclear deformation, dynamic loading enhances the convective transport of mobile solutes, especially larger molecules such as ADP and growth factors. Fluid flow that involves the convection of mobile counter-ions past ionized charge groups on immobilized macromolecules generates streaming potentials (5).
Cell proliferation was significantly greater in pre-cultured chondrocyte/agarose constructs. The samples were left to freely swell for 24 hours upon seeding. The newly formed pericellular matrix around chondrocytes within agarose has a higher elastic modulus than agarose/chondrocyte constructs (13), which should influence the mechanical response of the cell. Furthermore, in support of the current study, Buschmann et al. (5) demonstrated that the readiness of the cells occurs within one day, as determined by hyaluronan and integrin assays.
As suggested by Hunter et al. (11), the mechanical signals transmitted to the cells may vary substantially between different scaffolding environments. Chondrocytes in native tissue bind to the ECM via cell adhesion, which can transfer matrix strains through mechanosensitive ion channels directly to the cytoskeleton, whereas in agarose, the cells adhere to polysaccharide molecules, making pre-culture advantageous when polysaccharide matrices such as agarose or alginate are used. With pre-culturing, the cells are able to bind to the new pericellular matrix as it is deposited, thus providing a biomechanical interaction between the pericellular environment and the chondrocytes (11,32).
Hunter et al. (11) suggested that stimulation interspersed with periods of rest might enhance tissue formation. This resting period is essential to permit the restoration of cell sensitivity because continued compression is thought to cause mechanosensory saturation that would, in turn, decrease the sensitivity of the cells to additional mechanical stimuli (11,33).
As shown in Figure 2B, the chondrocytes subjected to sinusoidal bi-axial loading demonstrated a marked reduction in DNA content compared with the unstrained control samples. Considering the multiple phases of the cell cycle, the cells might take a relatively long time to complete one cycle. Freshly isolated chondrocytes are highly metabolically active until they have had time to deposit some of their own matrix (34). Previous studies by Waldman et al. (10,35) have shown that intermittent multi-axial loading on chondrocytes resulted in a decrease in the total DNA content of stimulated samples, which may have been caused by either cell death or cell cycle inhibition. An early study revealed that cell viability and cell cycle progression were shown to be initially inhibited by mechanical stimulation. However, once a proper ECM is built, mechanical stimulation begins to have positive effects. In fact, in a study by Huselstein et al. (36), the positive effect was only seen after 21 days.
In summary, chondrocytes seeded in three-dimensional scaffolds and subjected to bi-axial loading (i.e., superimposed compressive and shear strain) synthesize increased levels of matrix when compared with chondrocytes subjected to only compression. In agreement with previous studies, this study also shows that the pre-culturing of chondrocytes is an important prerequisite for effective three-dimensional culture, specifically in terms of cell proliferation. However, there is still a need to investigate the effect of a longer period of pre-culture. Furthermore, the effects of variations in the loading parameters, including changes in the frequency, duration, amplitude and waveform applied during the loading period, as well as the effects of shear loading in isolation, remain unknown.
This study was funded by the Ministry of Higher Education of Malaysia (UMRG: RG033/09AET and PPP: PS109/2010A). The authors would like to thank Mr. Adhli Iskandar for logistical support in the acquisition of the source tissue.
No potential conflict of interest was reported.
Nawi I contributed substantially to the conception and design of the study, data acquisition, analysis and interpretation, and manuscript draft. Pingguan-Murphy B revised the manuscript and approved the final version of the manuscript.