The role of structural brain changes and their correlations with neuropsychiatric symptoms and disability in Alzheimer's disease are still poorly understood.
OBJECTIVE:To establish whether structural changes in grey matter volume in patients with mild Alzheimer's disease are associated with neuropsychiatric symptoms and disability.
METHODS:Nineteen Alzheimer's disease patients (9 females; total mean age = 75.2 y old ±4.7; total mean education level = 8.5 y ±4.9) underwent a magnetic resonance imaging (MRI) examination and voxel-based morphometry analysis. T1-weighted images were spatially normalized and segmented. Grey matter images were smoothed and analyzed using a multiple regression design. The results were corrected for multiple comparisons. The Neuropsychiatric Inventory was used to evaluate the neuropsychiatric symptoms, and the Functional Activities Questionnaire and Disability Assessment for Dementia were used for functional evaluation.
RESULTS:A significant negative correlation was found between the bilateral middle frontal gyri, left inferior temporal gyrus, right orbitofrontal gyrus, and Neuropsychiatric Inventory scores. A negative correlation was found between bilateral middle temporal gyri, left hippocampus, bilateral fusiform gyri, and the Functional Activities Questionnaire. There was a positive correlation between the right amygdala, bilateral fusiform gyri, right anterior insula, left inferior and middle temporal gyri, right superior temporal gyrus, and Disability Assessment for Dementia scores.
CONCLUSIONS:The results suggest that the neuropsychiatric symptoms observed in Alzheimer's disease patients could be mainly due to frontal structural abnormalities, whereas disability could be associated with reductions in temporal structures.
The worldwide projection of Alzheimer's disease (AD) prevalence predicts that there will be approximately 106 million patients by 2050.1 Dementia is one of the main causes of elderly cognitive decline and functional impairment,2 leading to disastrous consequences for patients and their relatives in regard to the quality of life, and it also causes immense expense for the healthcare system.3 The neuropsychiatric symptoms, another highly prevalent problem even in the predementia phase,4,5 are also correlated with additional adverse events for patients, families, and caregivers, such as faster cognitive decline, earlier institutionalization, and higher mortality.6
Clinical evidence suggests that AD patients with different groups of neuropsychiatric symptoms have a heterogeneous clinical evolution.7 AD patients with behavior disorders might have different pathological features when compared with AD patients without them.8 Previous studies have shown that neuropsychiatric symptoms and cognitive function in AD patients might have separate structural pathologies.9 Depression and anxiety can precede in years the development of cognitive decline in AD.10
More specific regional associations with a range of behavioral symptoms have been identified using different neuroimaging modalities. Apathy was associated with frontal structures;11,12 delusion was correlated with frontal, parietal, and temporal structures;13 depressive symptoms with thalamus, lentiform nucleus, and medial temporal cortex;14 and agitation was associated with temporal and frontal structures.12 Even with several studies, divergence of opinion still remains as to whether cognition and behavior are independent and heterogeneous dimensions or whether behavior disorders are an inevitable and nonspecific consequence in dementias.15,16 Few studies have investigated the neuropathological mechanisms of functional decline in AD. One study correlated disability with frontal, temporal, and occipital structures.17 In addition, few studies have explored the correlation between anatomical changes, neuropsychiatric symptoms, and functional losses, especially in patients in developing countries.
This study was designed to clarify whether voxel-based morphometry structural changes in grey matter volume in patients with mild AD are associated with neuropsychiatric symptoms and functional impairment.
METHODSSubjectsNineteen patients with mild AD (9 female) were recruited from a multidisciplinary memory clinic. Their mean age was 75.2 y (SD = 4.7, range 66–86), and their mean education level was 8.5 y (SD = 4.9, range 3–19). All patients in the study fulfilled the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria for probable Alzheimer's disease.
Exclusion criteria included significant symptoms of depression (Geriatric Depression Scale > 6), Functional Assessment Staging <3 or >4, significant radiological evidence of ischemic brain disease (Modified Hachinski Ischemia Score >4), previous cerebrovascular event, Mini-Mental State Examination score <20, or evidence of other degenerative or secondary dementias. Other exclusion criteria included end-stage chronic disease or unstable medical condition, premorbid psychiatric history, antipsychotic or psychoactive medication dose adjustments in the last 2 mo before study enrolment, initial treatment with cholinesterase inhibitors within the 2 mo before study enrolment, significant visual or hearing impairment, age <60 y, and any other condition that could prevent the patient from undergoing a magnetic resonance imaging (MRI) examination. Highly symptomatic depressive patients were excluded, to avoid bias in cognitive assessment. Their performance on this evaluation is strongly influenced by the level of depressive symptoms.18
ProceduresEach patient and his or her caregiver had a complete interview with a consultant geriatrician that included assessment of demographic and medical conditions including tobacco use, diabetes mellitus, number of medications used on a daily basis, body mass index, waist-to-hip ratio, number of psychoactive medications, and period of use of cholinesterase inhibitors. The functional status assessment was performed using the Pfeffer Functional Activities Questionnaire, which ranged from 0 points (completely independent) to 30 points (completely dependent), and the Disability Assessment for Dementia, which ranged from 0% (completely dependent) to 100% (completely independent). The neuropsychiatric symptoms were evaluated by the Neuropsychiatric Inventory, which ranged from 0 points (no behavioral problems) to 144 points (maximum of neuropsychiatric symptoms). Each participant also underwent a cognitive evaluation and had an MRI brain scan taken. The Neuropsychiatric Inventory, Disability Assessment for Dementia and Functional Activities Questionnaire values were then correlated with grey matter volume values extracted from the patients' MRI brain scans.
The Ethics Committee of the Universidade Federal de São Paulo and Hospital Santa Marceline approved the study, and informed consent was obtained from all participants (and their relative or guardian or caregiver) prior to inclusion in the study.
MRI data acquisition, analysis, and postprocessingExamination of the brain was performed for all subjects using a 1.5 T [Magnetom Sonata (Maestro Class); Siemens AG, Erlangen, Germany] using an eight-channel head coil. To minimize variation in head position, subjects were positioned by the same investigator using the orbitomeatal line as a landmark. Two conventional sequences were performed: (a) Axial T2-weighted fuid-attenuated inversion recovery in a plane parallel to the anterior commissure-posterior commissure (AC—PC) line [TR (repetition time) = 8,500 ms, TE (echo time) = 107 ms, TI (inversion time) = 2,500 ms, slice thickness = 5.0 mm, slice interval = 1.5 mm, FOV (field of view) = 240 mm, matrix size = 256×256, number of excitations (NEX) = 1]; and (b) Sagittal T1-gradient echo volumetric acquisition for multiplanar reconstruction (TR = 2,000 ms, TE = 3.42 ms, flip angle = 15, FOV = 256 mm, 1.0 mm slice thickness with no gaps, totaling 160 slices per slab, matrix size = 256×256, NEX = 1).
This study used the VBM5 toolbox, which utilizes and extends the new unified segmentation approach19 implemented in Statistical Parametric Mapping (SPM5), executed in Matlab 7.0. The sagittal T1 DICOM files were converted to NIFTI-1 format. The converted files were then segmented into grey and white matter and normalized using the unified model cited above. Voxel values were modulated by Jacobian determinants derived from the spatial normalization, thus allowing brain structures with decreased volumes after spatial normalization to have their total counts decreased by an amount proportional to the degree of volume discounted. The final voxel resolution after normalization was 1 mm.3 The obtained grey matter images were finally smoothed with a Gaussian filter at full width at the maximum height equal to 8 mm and entered into statistical analysis. The sum of all voxel values within the segmented images, in native space, approximates intracranial volume within the corresponding partition. Intracranial volume was computed from the sum of grey, white, and cerebral spinal fluid volume.
Statistical analysisDemographic, clinical, cognitive, functional, and behavioral data were analyzed with SPSS 13.0 (SPSS, Chicago, IL). Prior to conducting analyses, measurements were examined for normality using the Shapiro-Wilk test. The association between the Neuropsychiatric Inventory and Disability Assessment for Dementia and Functional Activities Questionnaire scores was evaluated by Pearson's correlation coefficient or Spearman's rho, when appropriate. The level of statistical significance was set at p<0.05.
The correlations between regional grey matter volume and the Neuropsychiatric Inventory, Functional Activities Questionnaire, and Disability Assessment for Dementia scores in AD patients were evaluated using the voxel-based morphometry multiple-regression model. Brain volume and age were used in the model as covariates. Resulting clusters were reported as significant at a p<0.001 level, two-tailed, uncorrected for multiple comparisons. A small volume correction was applied when there was a strict a priori hypothesis that was already implicated in AD pathophysiology (hippocampus, entorhinal and perirhinal cortices, temporal gyri) emerging from whole brain analyses. We performed a small volume correction, placing a sphere with a 5-mm radius centerd at the local maxima, which was equivalent to a volume of 500 mm,3 with a threshold of p<0.01, corrected for multiple comparisons using the False Discovery Rate. Unpredicted findings were considered to be significant only if they survived small volume correction for multiple comparisons (p<0.05).
RESULTSDemographic, clinical, cognitive, functional, and behavioral dataThe data description of participants is fully detailed in Table 1.
Description of demographic, clinical, cognitive, functional, and behavioral data.
| Variable | Mean | SD; range | Median |
|---|---|---|---|
| Age (years) | 75.2 | 4.7; 66–86 | 74 |
| Education (years) | 8.5 | 4.9; 3–19 | 8 |
| Time of cholinesterase inhibitor use (months) | 41.9 | 26; 4–106 | 36 |
| Mini Mental State Examination | 24.21 | 2.78; 20-29 | 24 |
| Verbal fluency for letters F, A, and S | 22.15 | 11.22; 8-56 | 19 |
| Verbal fluency for animals | 10.52 | 2.76; 6-16 | 11 |
| Clock drawing test | 6.10 | 2.67; 2-10 | 6 |
| Functional Activities Questionnaire | 8.89 | 4.5; 1-22 | 10 |
| Disability Assessment for Dementia | 89.36 | 9.96; 60-100 | 92 |
| Neuropsychiatric Inventory | 20.36 | 19.25; 0-77 | 16 |
The correlation analysis of functional and behavioral scales showed a significant association between the Disability Assessment for Dementia and Functional Activities Questionnaire (r = −0.862, p<0.001), Disability Assessment for Dementia and Neuropsychiatric Inventory (r = −0.475, p<0.014), and Neuropsychiatric Inventory and Functional Activities Questionnaire (r = 0.551, p<0.04).
The behavioral assessment showed that 17 out of 19 patients (89%) had at least one symptom in the behavioral assessment according to their caregivers. Irritability was the most frequent abnormal behavior (63%) observed in the Neuropsychiatric Inventory symptoms field, followed by apathy (47%), agitation (47%), delusions (42%), and euphoria (42%). The least frequent behavior was hallucination (10%).
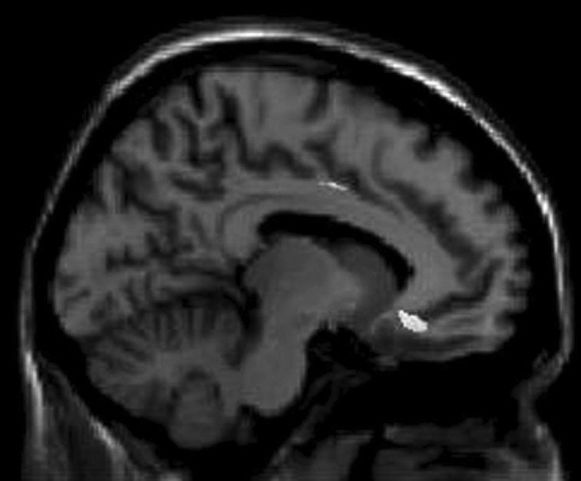
Neuroimaging findings and instrumental assessment correlationsThe voxel-based morphometry multiple-regression analysis showed that there were significant negative correlations between the grey matter volume of the bilateral middle frontal gyri, right orbitofrontal gyrus and left inferior temporal gyrus and total Neuropsychiatric Inventory scores (Figure 1).
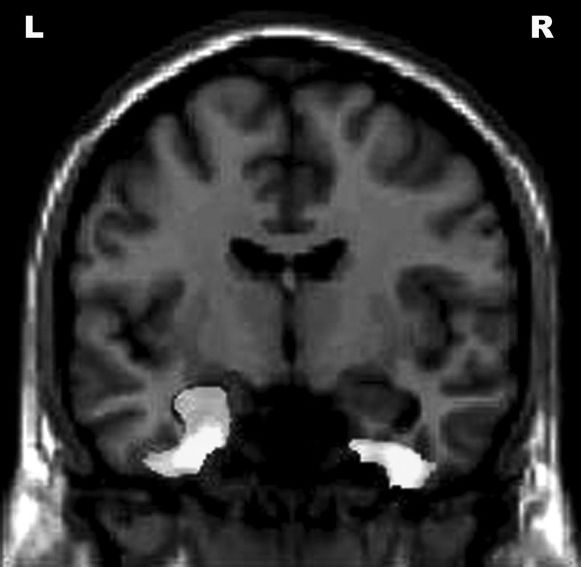
Negative correlations were found between the bilateral fusiform gyri, left hippocampus, bilateral middle temporal gyri, and Functional Activities Questionnaire scores (Figure 2).
Correlations between Functional Activities Questionnaire scores and reduced gray matter volume in different areas of the brain. The results are displayed at an uncorrected p<0.001 level and plotted onto the SPM5 canonical T1 image for the purpose of illustration. R = right; L = left.
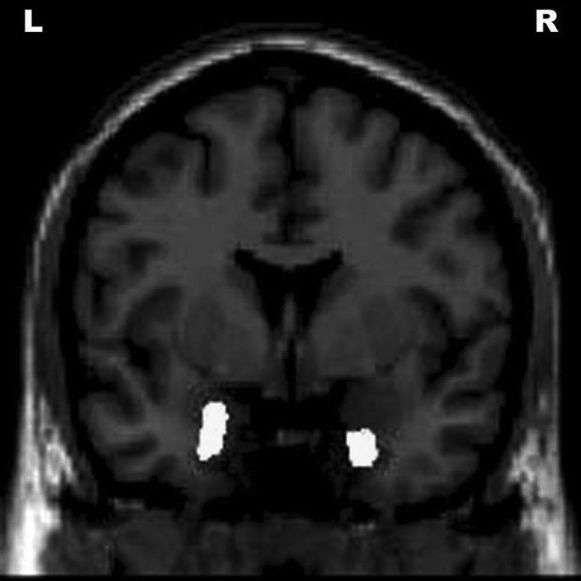
Positive correlations were seen between the right amygdala, bilateral fusiform gyri, right anterior insula, right superior temporal gyrus, left inferior temporal gyrus, left middle temporal gyrus, and Disability Assessment for Dementia scores (Figure 3).
A positive correlation was also observed between volume of frontal and temporal brain structures and cognitive tests. The detailed correlation analysis is described in Table 2.
Neuroimaging findings, cognitive, behavioral, and functional scores correlations.
| Instrument[Positive (PC) ornegative (NC)]correlation ] | Brain region (R = right, L = left) | MNIcoordinates | KE | Z-score |
|---|---|---|---|---|
| MMSE [PC] | R Inferior temporal gyrus | 56 -2 -36 | 190 | 3.64 |
| L Inferior temporal gyrus | -45 1 -40 | 259 | 3.51 | |
| L Fusiform gyrus | -36 -12 -41 | 61 | 3.27 | |
| L Insula | -35 -12 -5 | 94 | 3.26 | |
| FAS [PC] | L Inferior frontal gyrus | -46 12 18 | 473 | 3.63 |
| CDT [PC] | R Putamen | 22 16 -1 | 786 | 3.51 |
| L Putamen | -20 17 0 | 39 | 3.23 | |
| L Insula | -40 20 11 | 200 | 3.99 | |
| R Insula | 41 20 -11 | 70 | 3.50 | |
| NPI [NC] | ||||
| R Middle frontal gyrus | 43 52 -5 | 4493 | 4.34 | |
| L Middle frontal gyrus | -32 57 -12 | 12260 | 3.76 | |
| R Orbitofrontal gyrus | 9 50 -24 | 4493 | 74.32 | |
| L Inferior temporal gyrus | -48 -59 -19 | 19433 | 3.65 | |
| FAQ [NC] | ||||
| R Fusiform gyrus | 39 -12 -39 | 4914 | 5.01 | |
| L Fusiform gyrus | -27 -8 -35 | 9813 | 4.66 | |
| L Hippocampus | -34 -21 -15 | 9813 | 3.30 | |
| L Middle temporal gyrus | -57 -29 -9 | 9488 | 3.91 | |
| R Middle temporal gyrus | -31 20 -17 | 1769 | 3.51 | |
| DAD [PC] | ||||
| R Amygdala | 29 9 -28 | 4102 | 4.72 | |
| R Fusiform gyrus | 37 -17 -31 | 4102 | 4.11 | |
| L Fusiform gyrus | -32 -16 -32 | 2955 | 4.33 | |
| R Anterior insula | 42 -15 -1 | 2324 | 4.09 | |
| L Inferior temporal gyrus | -48 -59 -19 | 433 | 3.65 | |
| L Middle temporal gyrus | -60 -22 -8 | 929 | 4.38 | |
| R Superior temporal gyrus | 58 -6 -17 | 1237 | 3.98 |
PC: Positive correlation, NC: negative correlation, MMSE: Mini Mental State Examination, FAS: verbal fluency for letters F, A, and S, CDT: clock drawing test, FAQ: Functional Activities Questionnaire, DAD: Disability Assessment for Dementia,NPI: Neuropsychiatric Inventory, MNI: Montreal Neurologic Institute coordinates of peak effect, KE: voxel extent threshold, R: right, L: left. Results are reported at p<0.05 for small volume correction.
Neuropsychiatric symptoms were very prevalent in our sample of mild AD patients. Of these symptoms, irritability was the most frequent abnormal behavior, followed by apathy, agitation, delusions, and euphoria. These behavioral problems associated with AD tend to show a trajectory of increasing prevalence and severity over time, an attribute they share with cognitive and functional decline.4 Our paper confirms the high prevalence that was also seen in other studies.5,20 This different prevalence of some Neuropsychiatric Inventory domains could be explained by the exclusion of highly symptomatic depressive patients, the different dementia stage of our sample, differences in cultural manifestations of behavior, and sample size. A wide variation of the Functional Activities Questionnaire scores (1-22) was also observed. This assessment tool is based on caregiver judgement, generally influenced by the burden of care and cultural aspects of reported disability.21 We also observed a positive correlation between cognitive test scores and grey matter volume of frontal and temporal structures.
The voxel-based morphometry results showed significant negative correlations between the volume of frontal structures (middle frontal gyri and right orbitofrontal gyrus), temporal structure volume (left inferior temporal gyrus), and total Neuropsychiatric Inventory scores. Similar findings were also observed in previous studies, predominantly in the voxel-based morphometry field, molecular imaging techniques, and pathological studies, confirming the role of these structures in the genesis of neuropsychiatric symptoms. Symptoms like disinhibition have been associated with grey matter volume reduction in the right middle frontal gyri,22 depressive symptoms and bilateral atrophy of the medial orbitofrontal cortex,23 eating disorders with perfusion changes of the orbitofrontal cortex,24 agitation and aberrant motor behavior with greater neurofibrillary tangle pathology of the orbitofrontal cortex,25 apathy with left frontal structures,11 delusions and apathy with decreased grey matter volume density in the frontal lobe,12 neuropsychiatric symptoms with grey matter volume reduction of the right lateral middle frontal gyri and right orbitofrontal cortex,26 and neuropsychiatric symptoms with greater neurofibrillary tangle pathology of the orbitofrontal cortex.27
Significant correlations between the grey matter volume of temporal structures (fusiform gyri, hippocampus, amygdala, insula, temporal gyri) and functional impairment (negative correlation with the Functional Activities Questionnaire and positive correlation with Disability Assessment for Dementia) were also observed. Contrary to expectations, no correlation was found between disability and frontal structures. A possible explanation for these findings is that the functions generally assessed are dependent on executive functions and prospective memory impairment, closely related to temporal structures. Studies focusing on the neuroanatomical correlation of functional impairment in AD are rare. Disability in AD subjects was associated with a greater overall pathologic burden of the medial temporal, occipital, and orbital frontal regions,17 and decreased gray matter volume in the medial frontal and temporal-parietal cortices.28 Another study observed an association of decreased cerebral activity in the bilateral parietal and temporal cortices, precuneus, and left middle frontal gyrus and the dementia scales, including instrumental activities of daily living.29 Previous articles have demonstrated cognitive or behavioral (not functional) correlation between higher senile plaque density in the fusiform gyri and performance on visuoperceptual tests30 and insula pathology and behavioral abnormalities.31
The selective pathological involvement of some neocortical areas and ventromedial temporal lobe structures, which is common in AD,32 was also observed in our neuroimaging findings (middle frontal gyri, middle temporal gyri, and amygdala) and correlated with neuropsychiatric symptoms and the Neuropsychiatric Inventory. Bilateral grey matter volume reduction of the fusiform gyri and left middle temporal gyri correlated with both the Functional Activities Questionnaire and Disability Assessment for Dementia. The left inferior temporal gyrus was associated with both the Neuropsychiatric Inventory and Disability Assessment for Dementia.
Results should be interpreted as preliminary and with caution. A few limitations of the present study warrant mentioning. A small group of AD subjects were enrolled in the study with the absence of a control group for the clinical assessment. Even though the controls were submitted to an MRI scan, the same correlational analysis on the voxel-based morphometry could not be performed, because the Functional Activities Questionnaire, Disability Assessment for Dementia, and Neuropsychiatric Inventory scales were designed for dementia patients.
A deeper comprehension of pathophysiological mechanisms of behavioral and functional aspects of AD and their related anatomic changes may shed light on a more effective diagnostic, prognostic, and therapeutics approach.
CONCLUSIONSVoxel-based morphometry results suggest that the neuropsychiatric symptoms observed in these AD patients could be mainly associated with abnormalities in frontal structures, whereas functional impairment could be mainly associated with volume reductions in temporal structures.