Awake craniotomy has become a common procedure and its application has been continually evolving. Anesthesia for awake craniotomy poses a unique challenge to anesthesiologists. The aims of this article are to review under a critical perspective of the author, the current evidence and application of awake craniotomy and to briefly describe the principles of anesthetic management during this procedure.
La craneotomía en el paciente despierto se ha generalizado y su aplicación ha evolucionado continuamente. La anestesia para este procedimiento plantea un reto singular para los anestesiólogos. Son revisar, bajo una perspectiva crítica del autor, la evidencia actual y la aplicación de la craneotomía en el paciente despierto, y describir brevemente los principios del manejo anestésico durante el procedimiento.
Awake craniotomy poses a unique challenge to anesthesiologists, and its success is highly dependent on careful patient selection and the experience of the surgical and anesthesia team. The modern era of awake craniotomies began more than 50 years ago when Penfield and Andre Pasquet started to perform awake craniotomies for epileptic foci excision.1 Historically, anesthesia for awake craniotomy was regarded as a high-risk procedure and was only performed when it was absolutely indicated. With the improved understanding of cerebral localization and the availability of new anesthetic agents, the application of awake craniotomy has become much broader and safer than before. The aims of this article are to review the current evidence and application of awake craniotomy and to briefly describe the principles of anesthetic management during this procedure.
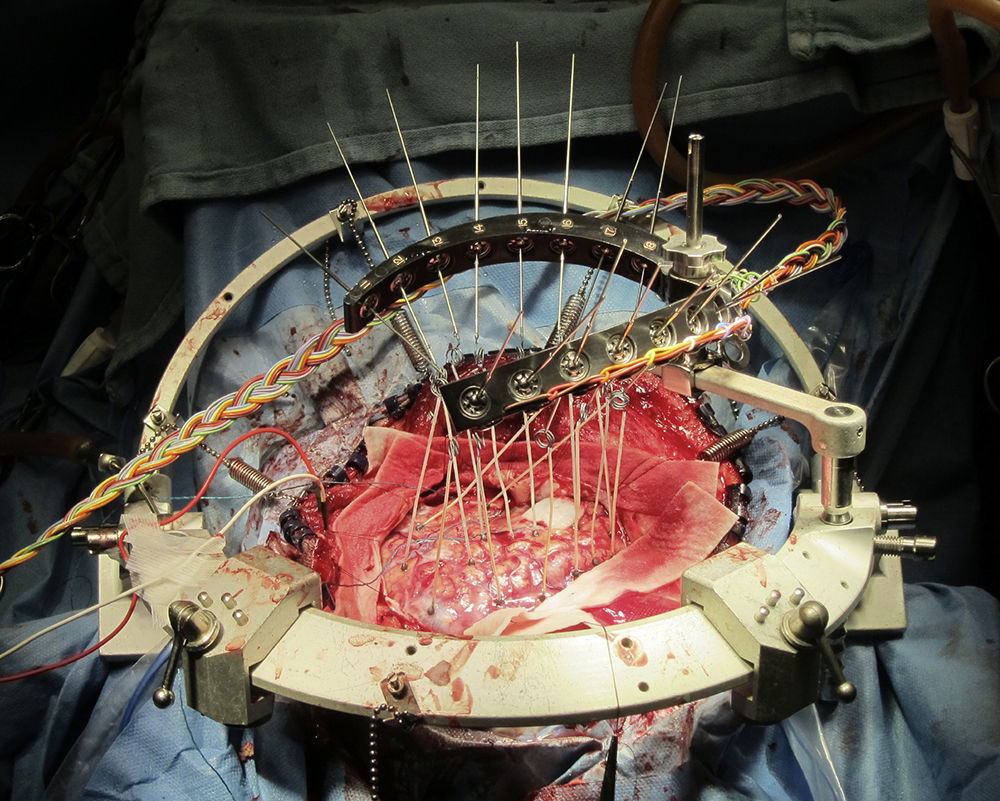
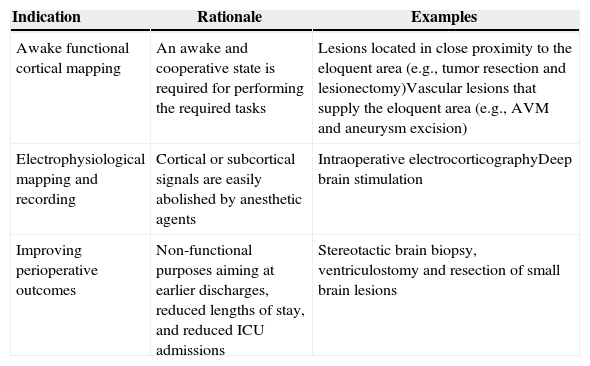
Indication for awake craniotomyThe current indications for awake craniotomy are listed in Table 1. The use of awake craniotomy with electrocorticography in surgical excision of intractable epileptic foci has been started by Penfield since 1950s.1 Electrocorticography is an invasive electrophysiological technique for the direct recording of the cortical potentials from the surface of the brain to localize seizure foci (Fig. 1). However, intraoperative electrocorticography is greatly affected by the anesthetic agents, and awake craniotomy is indicated to minimize pharmacological interference with the recordings.2 With the advancement of pre-surgical imaging techniques, the use of intraoperative electrocorticography for localization and hence the associated use of awake craniotomy for this purpose have been markedly reduced.
Current indications for awake craniotomy.
| Indication | Rationale | Examples |
|---|---|---|
| Awake functional cortical mapping | An awake and cooperative state is required for performing the required tasks | Lesions located in close proximity to the eloquent area (e.g., tumor resection and lesionectomy)Vascular lesions that supply the eloquent area (e.g., AVM and aneurysm excision) |
| Electrophysiological mapping and recording | Cortical or subcortical signals are easily abolished by anesthetic agents | Intraoperative electrocorticographyDeep brain stimulation |
| Improving perioperative outcomes | Non-functional purposes aiming at earlier discharges, reduced lengths of stay, and reduced ICU admissions | Stereotactic brain biopsy, ventriculostomy and resection of small brain lesions |
Currently, awake craniotomy is commonly indicated for procedures that require awake functional cortical mapping, where the lesion is located in close proximity to the eloquent cortical tissues that are indispensable for defined cortical functions. The motor, sensory, visual and language cortex have been successfully mapped during awake craniotomy.3,4 Tailored resection can be performed to preserve the patients’ function. A typical example of this category is a glioma resection in the motor or Broca's area.
The availabilities of propofol and ultrashort-acting opioids that permit rapid and smooth anesthetic control have led to the establishment of a wide application of awake craniotomy in neurosurgical patients, irrespective of the requirement for functional cortical mapping or electrophysiological recording. During the 1990s, awake craniotomy was advocated for ambulatory procedures.5 The indication for awake craniotomy has already been extended from procedures necessitating awake functional mapping to methods aiming to improve perioperative outcomes and to minimize resource utilization, including stereotactic brain biopsy, ventriculostomy and the resection of small brain lesions.5,6 In some centers, awake craniotomy has already been used routinely and non-selectively in the majority of patients for supratentorial tumor excision.7
Current evidence for awake craniotomyThe major benefit of awake craniotomy is to enable a tailored resection that can theoretically maximize the extent of the tumor resection and can minimize the neurological damage. The current supporting evidence is mainly based on prospective cohort and retrospective chart reviews.8 One of the recent large population cohort studies, involving 575 patients, has compared the gross total resection rate and postoperative neurological deficits between awake craniotomy and general anesthesia patients.9 Awake craniotomy was associated with a higher gross total resection rate in the eloquent area (37% vs 14%, p<0.05), fewer permanent neurological deficit (4.6% vs 16%, p<0.001) and fewer new-onset postoperative neurological deficits (3.3% vs 58% of patients, p<0.001). However, these benefits were not consistently demonstrated by other studies, particularly for the prevention of neurological deficits.8 Interestingly, the only available randomized controlled study on this topic revealed that general anesthesia yielded fewer and non-significant neurological deficits.10
The evidence supporting awake craniotomy in earlier hospital discharges are more consistent, and nearly all the previous studies have demonstrated shorter hospital stays for awake craniotomy patients. The additional benefits include shorter ICU stays, a shorter total length of stay, less resource utilization and high patient satisfaction.7,8
Perioperative managementPreoperative preparationCareful patient selection is one of the major components of successful awake craniotomy. The selection should be individualized and based on the airway assessment, risks of sedation failure, patient's cooperation and the risks of intraoperative surgical complications such as bleeding. A history of alcohol or drug abuse, chronic pain disorders, low tolerance to pain and anxiety or psychiatric disorders are known risk factors for sedation failure.11 The selection of patients presenting with seizures or mixed dysphasia, having a history of using multiple anti-convulsants or undergoing perioperative loading of phenytoin is associated with the loss of intraoperative cooperation and results in failed awake craniotomies.12 Well-motivated and mature patients are the best candidates for awake craniotomy. There was also a recent pilot study of using objective psychological and psychophysiological test to select their patients.13 Although there are reports of the safe use of awake craniotomy in pediatric patients, the case selection should be more rigorous and based on the patients’ maturity and the individual risk-benefit assessment.14
Preoperative psychological preparation and rapport building are the important components of preoperative preparation. The preoperative discussion should include a realistic description of the entire procedure, the expected discomforts, the level of cooperation desired, the tasks that must be performed and the possibility of adverse events. A well-conducted preoperative consultation usually can alleviate the patient's anxiety and improve his or her cooperation during the awake craniotomy.
Intraoperative managementThere are three major anesthetic challenges for awake craniotomy: (i) provision of a rapid and smooth transition of the anesthetic depth according to the different surgical stages, (ii) maintenance of stable cerebral hemodynamic and cardiopulmonary function, and (iii) crisis management for an awake patient with an open cranium. The requirement regarding the depth of anesthesia varies markedly at the different stages of surgery. Over-sedation may lead to apnea, hypoxemia, hypercapnia and cerebral swelling, whereas under-sedation may result in agitation, arterial hypertension and tachycardia. The common anesthetic goals for all neurosurgical patients, such as the avoidance of hypercapnia and hypoxemia, adequate cerebral perfusion pressure and brain relaxation, may not be easily achieved in awake craniotomy patients with uncontrolled airways.
Adequate local anesthesia can usually be achieved by either using a scalp block or a regional field block. Arterial line is commonly used; but other invasive monitoring, such as central venous lines, is not routinely required. Comfortable positioning is mandatory because extremely limited movements will be possible after the head-pinning. The draping should always allow easy access to the patient's face and airway. A microphone may be used to facilitate communication with the staff.
Choice of anesthetic techniquesVarious institutions and anesthesiologists have their own favored techniques for awake craniotomies, including local anesthesia, conscious sedation, asleep-awake-asleep techniques and asleep-awake techniques. None of these techniques has any demonstrated superiority. In the asleep-awake-asleep techniques, the patient is under general anesthesia, with the use of a laryngeal mask airway or endotracheal intubation during the positioning, head-pinning and craniotomy. The general anesthesia is discontinued for the period of functional cortical mapping and intraoperative electrocorticography. After the complete resection, the patient is put back under general anesthesia for the skin closure.15 In the asleep-awake technique, the patient is kept awake throughout the rest of the procedure, with the flexibility of permitting additional mapping if symptoms develop.16 By contrast, for conscious sedation, the patient is maintained with spontaneous breathing throughout the entire procedure, and the sedatives and analgesics are titrated based on the surgical stages.
Choice of anesthetic agentsThe choice of anesthetic agents for awake craniotomy is highly dependent upon the requirement for functional cortical mapping and intraoperative electrocorticography. Intraoperative electrocorticography recordings are highly affected by different anesthetics, and the choice of anesthetic agents should have minimal effects on the suppression and over-activation. Complete cessation of all anesthetic agents for 20–30min prior to the electrocorticography has been recommended; however, this recommendation may not be feasible in all cases of awake craniotomy, and general anesthesia may even be necessary in some patients. A detailed discussion of the effects of anesthetics on electrocorticography is beyond the scope of this article, and readers can refer to other papers for references.4
The anesthetic goal for awake cortical mapping is to maintain an awake and cooperative patient for performing the required tasks. The purpose of the mapping procedure is to reliably identify the cortical areas and subcortical pathways involved in the motor, sensory, language, and cognitive functions. Inaccurate mapping could create a false sense of security with incorrect resection margins. Observation of the patient's reaction to the trans-cortical stimulation is used to define the function of the testing area. Clonic movement signifies primary motor area stimulation, whereas tonic movement is more related to the premotor area. For the mapping of language and cognitive functions, a fully awake and compliant patient is crucial for performing naming tasks and basic mental gymnastics, such as thinking of items within a category. Induced-inhibition of speech, language and cognition should be distinguished from non-compliance, over-sedation and focal seizures.2 During the mapping, the patient might feel uncomfortable and anxious due to experiencing involuntary movements or the inhibition of voluntary movements or language. The level of anesthetic depth should strike a balance between the patient's comfort and the accuracy of the mapping result.
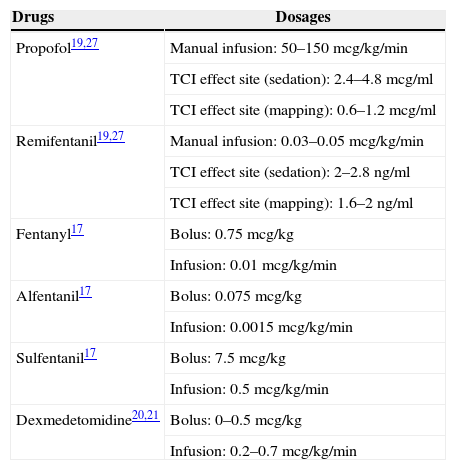
The most commonly used agents and dosages for awake craniotomy are listed in Table 2. Propofol infusion with a supplementary opioid is the most commonly reported choice for awake craniotomy. Propofol-only anesthesia with spontaneously breathing patients has been described as safe.17 The addition of an opioid is a common practice to improve the analgesic quality and reduce the hypnotic requirement. A comparison of different types of opioids, including fentanyl, alfentanil, or sulfentanil, found no significant difference in the operative condition, electrocorticography and stimulation testing.18 In a comparison of continuous remifentanil infusion or intermittent fentanyl, both narcotics yielded similar patient satisfaction, recall, and intraoperative complications, although fewer patients experienced reversible respiratory depression with the use of remifentanil.19 Therefore, all narcotics are regarded as equally good for use during awake craniotomy. Notably, the time of emergence from sedation for intraoperative testing with the use of remifentanil-propofol is approximately 9min.20
Commonly used anesthetic agents and dosages for awake craniotomy.
| Drugs | Dosages |
|---|---|
| Propofol19,27 | Manual infusion: 50–150mcg/kg/min |
| TCI effect site (sedation): 2.4–4.8mcg/ml | |
| TCI effect site (mapping): 0.6–1.2mcg/ml | |
| Remifentanil19,27 | Manual infusion: 0.03–0.05mcg/kg/min |
| TCI effect site (sedation): 2–2.8ng/ml | |
| TCI effect site (mapping): 1.6–2ng/ml | |
| Fentanyl17 | Bolus: 0.75mcg/kg |
| Infusion: 0.01mcg/kg/min | |
| Alfentanil17 | Bolus: 0.075mcg/kg |
| Infusion: 0.0015mcg/kg/min | |
| Sulfentanil17 | Bolus: 7.5mcg/kg |
| Infusion: 0.5mcg/kg/min | |
| Dexmedetomidine20,21 | Bolus: 0–0.5mcg/kg |
| Infusion: 0.2–0.7mcg/kg/min |
The ¿2 agonist dexmedetomidine has gained popularity for use in awake craniotomy owing to its unique ano-analgesic property, less dis-inhibition and minimal respiratory depressant effects. There is an increasing trend of using the combination of propofol and dexmedetomidine to minimize dis-inhibition and to ensure speedy awakening. Dexmedetomidine has also been demonstrated to have minimal effects on the electrocorticography and has been successfully used in awake craniotomy for epileptic surgery.21,22 However, there have been reports of more intense efforts being required to rouse the patient from dexmedetomidine sedation (i.e., via a physical stimulus such as sternal rubbing and calling of the patient's name). However, once roused and engaged, the patient remains able to cooperate with the cognitive testing.23 A direct comparison between dexmedetomidine and the propofol-remifentanil combination, particularly for performing awake cortical mapping, has yet to be evaluated.
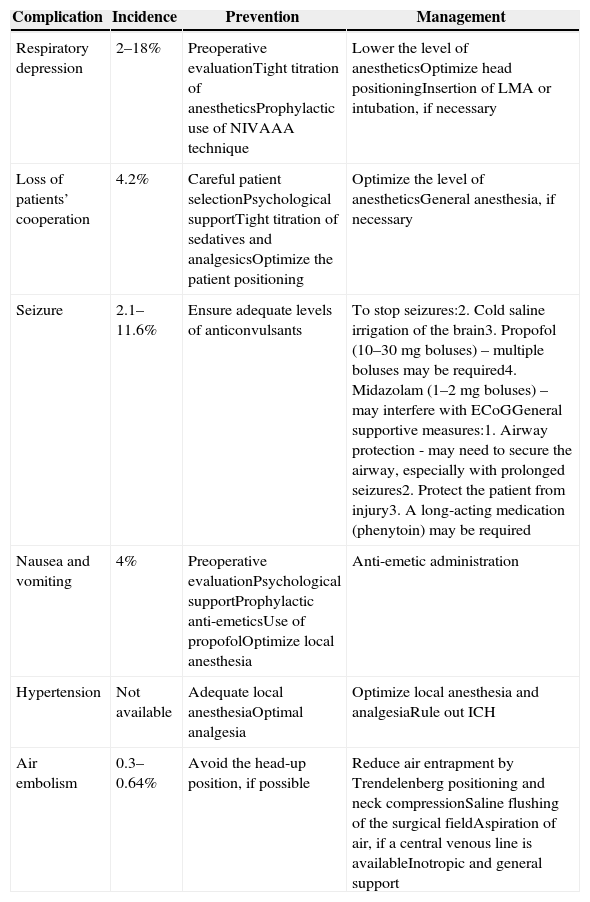
Complications and the safe performance of awake craniotomyMajor intraoperative complications include seizures, respiratory depression, air embolisms, cerebral swelling and the trigeminocardiac reflex. The common complications, preventive measures and management techniques are listed in Table 3. The overall complication rate is reported to be approximately 16.5%,5 with 6.4% patients failing to complete the mapping procedure.12 The main causes of failure are the onset of seizures and the loss of the patient's cooperation due to severe somnolence, restlessness or the development of mixed dysphasia. Failed awake craniotomies are associated with a lower incidence of gross-total resections, increased postoperative speech deterioration and a longer length of stay.12
Intraoperative complications of awake craniotomy.16,28,29
| Complication | Incidence | Prevention | Management |
|---|---|---|---|
| Respiratory depression | 2–18% | Preoperative evaluationTight titration of anestheticsProphylactic use of NIVAAA technique | Lower the level of anestheticsOptimize head positioningInsertion of LMA or intubation, if necessary |
| Loss of patients’ cooperation | 4.2% | Careful patient selectionPsychological supportTight titration of sedatives and analgesicsOptimize the patient positioning | Optimize the level of anestheticsGeneral anesthesia, if necessary |
| Seizure | 2.1–11.6% | Ensure adequate levels of anticonvulsants | To stop seizures:2. Cold saline irrigation of the brain3. Propofol (10–30mg boluses) – multiple boluses may be required4. Midazolam (1–2mg boluses) – may interfere with ECoGGeneral supportive measures:1. Airway protection - may need to secure the airway, especially with prolonged seizures2. Protect the patient from injury3. A long-acting medication (phenytoin) may be required |
| Nausea and vomiting | 4% | Preoperative evaluationPsychological supportProphylactic anti-emeticsUse of propofolOptimize local anesthesia | Anti-emetic administration |
| Hypertension | Not available | Adequate local anesthesiaOptimal analgesia | Optimize local anesthesia and analgesiaRule out ICH |
| Air embolism | 0.3–0.64% | Avoid the head-up position, if possible | Reduce air entrapment by Trendelenberg positioning and neck compressionSaline flushing of the surgical fieldAspiration of air, if a central venous line is availableInotropic and general support |
Abbreviation: NIV: non-invasive ventilation; AAA: asleep–awake–asleep techniques; LMA: laryngeal mask; EcoG: electrocorticography; ICH: intra-cranial hemorrhage.
Because intraoperative complications are not uncommon, certain preventive measures and monitoring are mandatory for the safe performance of awake craniotomy. In addition to all routine monitoring, monitoring of breathing both clinically and via end-tidal CO2 are mandatory for all patients. Optimizing the positioning and access are essential for easily controlling the airway, dealing with emergencies and communicating with patients during the mapping procedure. Intubation equipment, such as laryngeal mask, endotracheal tube, laryngoscopy, and medications should be prepared for emergency use. Fiber-optic bronchoscopy should also be easily accessible in the operation theater complex. Additionally, intraoperative seizure is a known complication during awake craniotomy. The majority of intraoperative seizures are mostly focal seizures related to cortical stimulation, and these seizures usually resolve spontaneously after the cessation of stimulation. However, ice-cold saline applied by the surgeon to the brain surface and small doses of anti-convulsants (e.g. 1mg/kg of propofol) may required in some cases.4
Protocols of outpatient awake craniotomyAmbulatory awake craniotomy has been performed and studied extensively in Toronto since the early 1990s. The initial successful same-day discharge rate was reported to be 89.1%,24 and the data of the subsequent two cohort studies from the same center were maintained as high as 92.4 and 94%, respectively.25,26 Their protocol emphasizes rigorous patient selection, minimally invasive surgical and anesthetic techniques and stringent discharge procedures. All patients are monitored for at least 6h after surgery with a routine pre-discharge CT scan and are assessed individually by a surgeon and anesthesiologist for the suitability of discharge. The patients are also followed up by home-care nurses and educated about the warning signs associated with serious complications such as ICH.
ConclusionsThe application of awake craniotomy has been continually evolving. Attention to every component, including careful patient selection, preoperative psychological preparation, solid rapport building, ensuring patient comfort in positioning, superb regional anesthesia, the proper choice of anesthetic techniques and agents, preparation and prompt crisis management, and continuous team communication, are the keys to successful awake craniotomy. Tight anesthetic control and the meticulous performance of mapping procedures are essential to achieve the highest accuracy of cerebral localization results. Finally, because complications are common during awake craniotomy, prompt crisis management, team communication and experienced anesthetic and surgical teams are important to maximize the utility and safety of this procedure.27–40
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Chui J. Anestesia para craneotomía en el paciente despierto: una actualización. Rev Colomb Anestesiol. 2015;43:22–28.