Advances in imaging, computing and optics have encouraged the application of minimally invasive surgical approach to a variety of neurosurgical procedures. The advantages include accurate localization of lesions usually inaccessible to conventional surgery, less trauma to healthy brain, blood vessels and nerves, shorter operating time, reduced blood loss, and early recovery and discharge. Nevertheless minimally invasive neurosurgical (MIN) procedures still have potential intra- and post-operative complications that can cause morbidity and mortality.
ObjectivesThe aim of this study was to review and analyze published literature describing experiences in the anesthetic management of the most commonly performed MIN procedures.
Materials and methodsNeurosurgical and neuroanesthesia literature (1990–2013) was reviewed and description of anesthetic technique/management and perioperative morbidity/mortality was reported. We also compared the different authors’ experience with MIN procedures.
ResultsThe neurosurgical literature dealing with MIN has expanded, but there are few references in relation to anesthetic management. Anesthesia goals remain the same: careful pre-operative assessment and planning, and meticulous cerebral hemodynamic control to ensure adequate cerebral perfusion pressure. The degree of postoperative care depends on local practice, patient factors and postoperative brain imaging.
Los avances en la formación de imágenes, la computación y la óptica han alentado la aplicación del enfoque quirúrgico mínimamente invasivo a una variedad de procedimientos neuroquirúrgicos. Las ventajas incluyen la localización exacta de las lesiones generalmente inaccesibles a la cirugía convencional, menos trauma al cerebro sano, vasos sanguíneos y nervios, más corto el tiempo de funcionamiento, la reducción de la pérdida de sangre, la recuperación temprana y el alta. Sin embargo los procedimientos neuroquirúrgicos mínimamente invasivos (NMI) todavía tienen potencial complicaciones intra y post-operatorias que pueden causar morbilidad y mortalidad.
ObjetivosEl objetivo de este estudio fue revisar y analizar la literatura publicada que describe las experiencias en el manejo anestésico de los procedimientos más comúnmente realizados en NMI.
Materiales y métodosLiteratura sobre neurocirugía y neuroanestesia (1990–2013). Revisión y descripción de la técnica anestésica/gestión y morbilidad perioperatoria/mortalidad notificada. Comparación de la experiencia de los diferentes autores en procedimientos de NMI.
ResultadosLa literatura sobre NMI se ha expandido, pero hay pocas referencias en relación con el manejo anestésico. Las metas anestésicas siguen siendo las mismas: la evaluación preoperatoria cuidadosa y la planificación, el meticuloso control de hemodinámica cerebral para asegurar la presión de perfusión cerebral adecuada. El grado de cuidado postoperatorio depende de la práctica local, factores del paciente y de imagen cerebral postoperatoria.
Neurosurgical literature dealing with minimally invasive neurosurgery (MIN) has expanded, but there are few references in relation to anesthetic management. Anesthesia goals remain the same: careful pre-operative assessment, planning, and meticulous cerebral hemodynamic control to ensure adequate cerebral perfusion pressure. Patients should be monitored as if undergoing a traditional craniotomy. It is important to select an anesthetic technique which allows rapid emergence for prompt neurological assessment.1
The following is a summary of anesthetic and perioperative features for the most commonly performed MIN procedures, after narrative review of literature about neurosurgery and neuroanesthesia between 1990 and 2013.
Closed brain biopsy“Closed” biopsy of the brain includes percutaneous frame-based (CT-guided with stereotactic frame), frameless (navigation-guided or stealth), and Mayfield 3-point skull pin fixation, with or without endoscopy.
Anesthetic considerationsThe choice of anesthesia for closed brain biopsy depends on the surgical technique, patient characteristics and positioning. Precordial Doppler can be used to detect venous air embolism in semi-sitting position.2 Neuronavigational procedures require a high degree of precision and patient immobility. This is easily achieved with general anesthesia and hence is the preferred technique over local anesthesia and sedation.3,4 In contrast to routine neuroanesthesia practice where a slack brain is desired, brain shift due to a “slack” brain may lead to loss of reliability of the navigational system. Maintenance of normocarbia and the avoidance of mannitol will minimize brain displacement. If a patient fails to awaken after the procedure, a CT scan should be performed to rule out cerebral edema, hematoma, or pneumocephalus.
When local anesthesia and monitored sedation is chosen, regional nerve blocks can be applied.5 Sedation may be required and achieved using dexmedetomidine, propofol, and/or remifentanil.2 An invasive, or non-invasive but continuous, blood pressure recording is useful to monitor for unexpected hypertensive peaks that could facilitate bleeding.
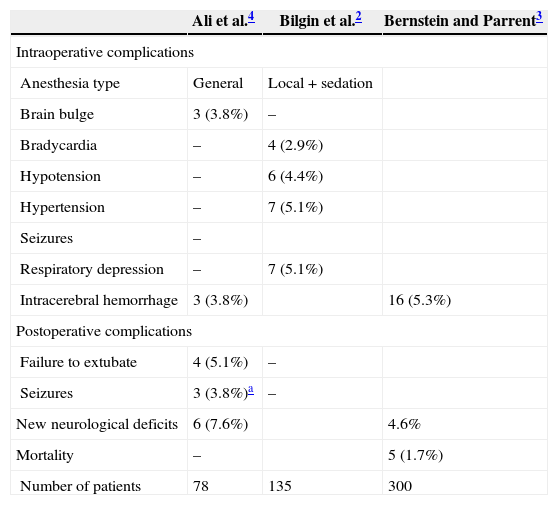
Perioperative settingTable 1 lists the perioperative complications associated with closed brain biopsy. The diagnostic yield, event rates, and biopsy-related mortality do not differ among the different closed biopsy techniques.4,6
Perioperative complications/mortality associated with closed brain biopsy.
| Ali et al.4 | Bilgin et al.2 | Bernstein and Parrent3 | |
|---|---|---|---|
| Intraoperative complications | |||
| Anesthesia type | General | Local+sedation | |
| Brain bulge | 3 (3.8%) | – | |
| Bradycardia | – | 4 (2.9%) | |
| Hypotension | – | 6 (4.4%) | |
| Hypertension | – | 7 (5.1%) | |
| Seizures | – | ||
| Respiratory depression | – | 7 (5.1%) | |
| Intracerebral hemorrhage | 3 (3.8%) | 16 (5.3%) | |
| Postoperative complications | |||
| Failure to extubate | 4 (5.1%) | – | |
| Seizures | 3 (3.8%)a | – | |
| New neurological deficits | 6 (7.6%) | 4.6% | |
| Mortality | – | 5 (1.7%) | |
| Number of patients | 78 | 135 | 300 |
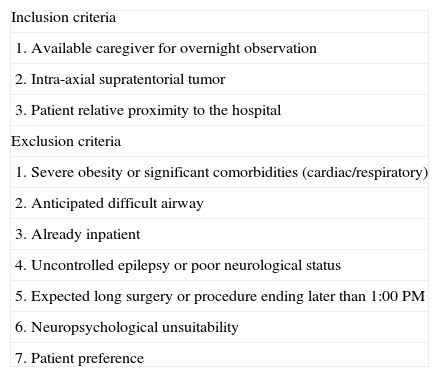
The degree of postoperative care varies between institutions. In Purzner et al.,7 of 244 patients who underwent a brain biopsy, 152 (62%) were selected to be outpatients; 143 patients (94.1%) were successfully discharged from the day surgery unit, 4.6% could not be discharged, and 1.3% were discharged and later readmitted (Table 2). Other centers perform a brain-imaging study before discharging patients from the postoperative care unit. Ideally, every center should have a clinical pathway that prepares and plans for closed brain biopsy patients depending on tumor location, edema, midline shift, the presence of intraoperative unexpected events and the center's historical results and outcomes.8
Inclusion/exclusion criteria for day surgery brain biopsy.
| Inclusion criteria |
| 1. Available caregiver for overnight observation |
| 2. Intra-axial supratentorial tumor |
| 3. Patient relative proximity to the hospital |
| Exclusion criteria |
| 1. Severe obesity or significant comorbidities (cardiac/respiratory) |
| 2. Anticipated difficult airway |
| 3. Already inpatient |
| 4. Uncontrolled epilepsy or poor neurological status |
| 5. Expected long surgery or procedure ending later than 1:00 PM |
| 6. Neuropsychological unsuitability |
| 7. Patient preference |
After surgery, patients are monitored in the postoperative care unit for 2h and then transferred to the day surgery unit for a minimum of 6h. A plain CT scan of the brain is performed 4h postoperatively. All patients are examined by the senior surgeon who determines fitness for discharge: normal postoperative CT, adequate pain control, wound hemostasis, stable neurological status, and the presence of a responsible adult who will monitor the patient overnight.
Deep brain stimulation (DBS) is the surgical treatment of choice for movement disorders, especially Parkinson's disease that are refractory to medical therapy. Intraoperative electrophysiological guidance consists of microelectrode recording, and macrostimulation in the awake patient to verify that the stimulation of the electrode improves the patient's symptoms with minimal side-effects.9 DBS has also been used to treat many other neurologic and psychiatric conditions including chronic pain, refractory epilepsy, obsessive compulsive disorder and depression. Frameless stereotactic systems have improved patient tolerance of these procedures.
Anesthetic considerationsThe main challenges are the effects of anesthetic agents on the nuclei microelectrode recording and the need for an awake, cooperative patient during intraoperative macrostimulation testing.9
DBS is usually performed with local anesthesia and monitored sedation. Propofol infusion can provide optimal sedation when patient cooperation is not needed. However, patients with Parkinson's disease are more sensitive to sedatives and are prone to airway obstruction and hypoventilation, especially if remifentanil is added. Dexmedetomidine may be the drug of choice, as it provides sedation with minimal respiratory depression.10 Once the electrodes are placed, the leads are tunneled and the pulse generator is inserted into a subcutaneous pocket below the clavicle, usually under general anesthesia.
General anesthesia may be the only option for patients with severe dystonia.11 Some centers are using intraoperative MRI guidance for insertion of the DBS device which then requires general anesthesia throughout the procedure.12,13
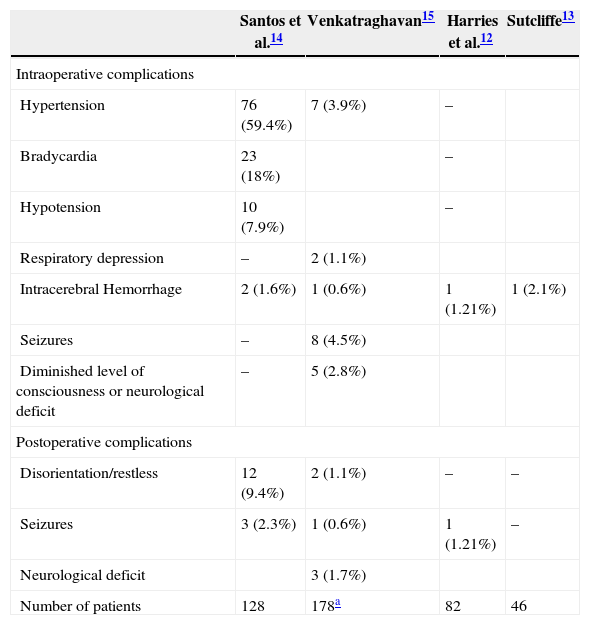
Perioperative settingTable 3 lists the perioperative complications associated with DBS for Parkinson's disease. Orthostatic hypotension may result from levodopa. On the other hand, hypertension during the procedure has been associated with increased risk of intracerebral hemorrhage.16 Intra-arterial blood pressure monitoring may be required in hypertensive patients and long lasting procedures. Bradycardia has been described during stimulation of the amygdala and hippocampus. Postoperative behavioral and cognitive problems are not uncommon after DBS insertion. Selective dopamine blockers (clozapine, quetiapine) may be used to treat postoperative hallucinations. Nonselective blockers (olanzapine, haloperidol) should be avoided.17 Withdrawal of medications that is necessary before DBS implantation can increase patients’ symptoms in the postoperative period and administration of anti-parkinsonian medication through a nasogastric tube may be necessary.18 Studies have shown that complications usually occur within 6h of surgery so patients may be discharged to the ward after a defined period of close observation. Some centers perform an imaging study before discharge to the neurosurgical ward.19
Perioperative complications associated with DBS for Parkinson's disease.
| Santos et al.14 | Venkatraghavan15 | Harries et al.12 | Sutcliffe13 | |
|---|---|---|---|---|
| Intraoperative complications | ||||
| Hypertension | 76 (59.4%) | 7 (3.9%) | – | |
| Bradycardia | 23 (18%) | – | ||
| Hypotension | 10 (7.9%) | – | ||
| Respiratory depression | – | 2 (1.1%) | ||
| Intracerebral Hemorrhage | 2 (1.6%) | 1 (0.6%) | 1 (1.21%) | 1 (2.1%) |
| Seizures | – | 8 (4.5%) | ||
| Diminished level of consciousness or neurological deficit | – | 5 (2.8%) | ||
| Postoperative complications | ||||
| Disorientation/restless | 12 (9.4%) | 2 (1.1%) | – | – |
| Seizures | 3 (2.3%) | 1 (0.6%) | 1 (1.21%) | – |
| Neurological deficit | 3 (1.7%) | |||
| Number of patients | 128 | 178a | 82 | 46 |
Endoscopic third ventriculostomy (ETV) is the treatment of choice for noncommunicating hydrocephalus. Neuroendoscopy has also been used to treat infective hydrocephalus and intraventricular hemorrhage. Other interventions include endoscopic removal of cysts, tumor biopsies, complete removal of intra and paraventricular tumors, intraventricular nontumoral lesions such as neurocysticercosis, hematomas, and choroid plexus cauterization in the treatment of hydrocephalus in developing countries.20,21
Anesthetic considerationsThe patient position for neuroendoscopy is usually supine with slight flexion of the neck, or with head up tilt ranging from 45° to 90°. The cranium is fixed in a head frame. Through a coronal burr hole, the endoscope is introduced into the ventricular system via the frontal horn. The endoscope has a channel for aspiration and a working channel through which a variety of instruments can be passed. The anesthesia goals are to ensure patient immobility, prevent, detect and treat sharp increases in intracranial pressure (ICP), and plan for rapid emergence for prompt neurologic assessment. Nitrous oxide is avoided to prevent its diffusion into air trapped in the ventricles and subdural space following decompression of the ventricles.22 Margetis et al. used preoperative intravenous corticosteroids to reduce the potential risk of chemical ventriculitis and subsequent hydrocephalus that may occur as a result of intraventricular spillage of colloid material during endoscopic resection.23
Warmed Lactated Ringer's solution is the most frequently used irrigation fluid.24–27 Salvador et al. found that the use of saline produced significant changes in CSF composition.28 To avoid intraoperative and postoperative complications arising from the use of irrigating fluids, care is taken to limit the loss of CSF and to use irrigation only when necessary.29
The fluid is usually infused under pressure and allowed to egress passively through an open port of the endoscope. Inadequate venting of the irrigating fluid will lead to marked increases in ICP.
In addition to standard monitors, intra-arterial monitoring is recommended.22,25–27 Continuous measurement of cerebral perfusion pressure is essential.26 To assess cerebral perfusion pressure (CPP), ICP can be measured indirectly by measuring the pressure inside the endoscope (PIN). PIN is obtained with a fluid-filled catheter connected to a stop-cock connected to the irrigation lumen of the neuroendoscope and attached to a pressure transducer zeroed at the skull base.30
It has been recommended that intraoperative CPP should be maintained above 40mmHg throughout.26 Kalmar et al. found that the occurrence of hypertension and tachycardia (“atypical Cushing reflex”) was the result of an increase in ICP. Also, the Cushing reflex developed in almost every case when cerebral perfusion pressure dropped below 15mmHg. However, the occurrence of bradycardia was not always associated with a low CPP.26
Middle cerebral artery blood flow velocity can be measured with a 2MHz transcranial Doppler (TCD) probe attached to the temporal window. Low CPP can occur precipitously with inadequate venting of the irrigation solution.24 However, maintaining a reliable TCD signal throughout surgery is technically difficult and sometimes unattainable.
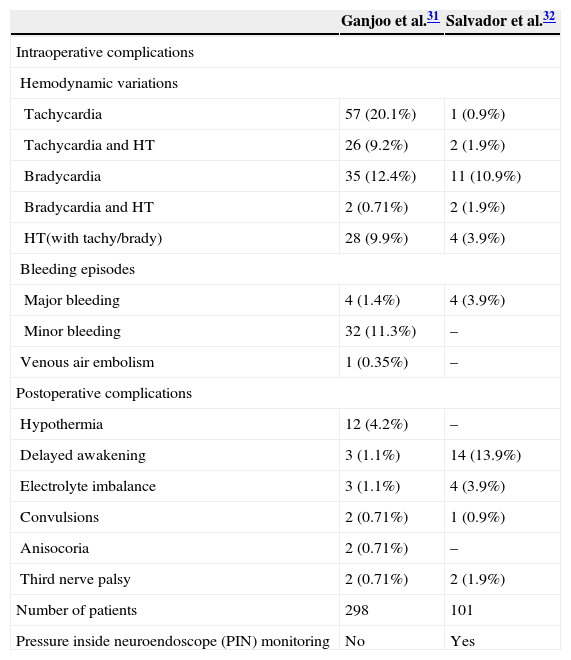
Perioperative settingTable 4 lists the perioperative complications.31,32 Cardiac arrhythmias are common and range from bradycardia, tachycardia, premature ventricular contractions, supraventricular tachycardia to asystole. Fortunately, they are often transient. The more serious complications include injury to brain structures, including the basilar artery and subsequent hemorrhage.
Perioperative complications associated with intraventricular neuroendoscopy.
| Ganjoo et al.31 | Salvador et al.32 | |
|---|---|---|
| Intraoperative complications | ||
| Hemodynamic variations | ||
| Tachycardia | 57 (20.1%) | 1 (0.9%) |
| Tachycardia and HT | 26 (9.2%) | 2 (1.9%) |
| Bradycardia | 35 (12.4%) | 11 (10.9%) |
| Bradycardia and HT | 2 (0.71%) | 2 (1.9%) |
| HT(with tachy/brady) | 28 (9.9%) | 4 (3.9%) |
| Bleeding episodes | ||
| Major bleeding | 4 (1.4%) | 4 (3.9%) |
| Minor bleeding | 32 (11.3%) | – |
| Venous air embolism | 1 (0.35%) | – |
| Postoperative complications | ||
| Hypothermia | 12 (4.2%) | – |
| Delayed awakening | 3 (1.1%) | 14 (13.9%) |
| Electrolyte imbalance | 3 (1.1%) | 4 (3.9%) |
| Convulsions | 2 (0.71%) | 1 (0.9%) |
| Anisocoria | 2 (0.71%) | – |
| Third nerve palsy | 2 (0.71%) | 2 (1.9%) |
| Number of patients | 298 | 101 |
| Pressure inside neuroendoscope (PIN) monitoring | No | Yes |
HT=hypertension.
Delayed awakening is a major concern to the anesthesiologist. Salvador et al. found a strong association between increased PIN over 30mmHg and increases in postoperative complications.32 Early detection and diagnosis of neurological dysfunction are imperative to exclude treatable causes such as intracranial hemorrhage.
Patients undergoing ETV may be managed as outpatients provided the procedure is short and uneventful.
Endoscopic endonasal pituitary surgeryEndoscopic endonasal surgery can be performed for both benign and malignant diseases of the cranial base. The advantages offered by this technique over conventional transcranial surgery are in part related to minimal disruption of normal tissue, superior visualization of the target, and the avoidance of brain and cranial nerve retraction.33 This approach is currently the preferred technique for resection of pituitary tumors. In a report of Dehdashti et al.,34 endoscopic pituitary adenoma resection was found safe and effective as the “traditional transsphenoidal” procedure. Comparable remission rates in functional tumors and a very low rate of complications were found.
Anesthetic considerationsPreoperative hormonal dysfunction needs to be carefully evaluated. Patients with acromegaly are at increased risk for cardiovascular disease including hypertension, coronary artery disease, cardiomyopathy, congestive heart failure, and arrhythmias due to effects of excess growth hormone secretion.35,36 Airway management and intubation may be challenging.37 It is safest to use awake intubating techniques when a difficult airway is suspected.38,39 Moreover up to 70% of acromegalic patients may have sleep apnea, which places them at increased risk for perioperative airway compromise.
Anesthesia goals and management are similar to those described for the classic transsphenoidal approach. Remifentanil in combination with propofol or a volatile agent provide hemodynamic stability, and allow for a more rapid recovery compared with volatile agents alone.40 In a study of 90 patients undergoing transsphenoidal pituitary surgery, patients were randomized to receive propofol, isoflurane or sevoflurane maintenance with anesthetic level titrated to bispectral index score between 40 and 60.41 Time to emergence and tracheal extubation were comparable in the propofol and sevoflurane groups. However, postoperative cognitive function, as measured by modified Aldrete score, at 5 and 10min was better in the patients who received propofol.
Following induction of anesthesia, local anesthetic containing epinephrine is injected into the nasal mucosa to prepare the nares for endoscopic surgery. Epinephrine may cause hypertension in patients with Cushing's disease or acromegaly, which maybe require treatment with ¿-blockers, or vasodilators such as nitroglycerin. Bilateral infraorbital nerve blockade with local anesthetic has been described to avoid the hypertensive response associated with surgical stimulation.42 A lumbar intrathecal catheter may be required to prevent or treat CSF leak. During endoscopy, neurosurgeons typically ask for permissive systemic hypotension, in addition to reverse Trendelenburg position, for improved optics in the surgical field. In this setting, regional cerebral saturation may be a useful tool to evaluate the maintenance of an adequate cerebral perfusion.43,44
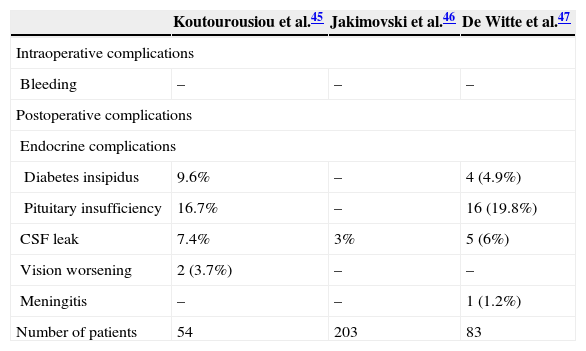
Perioperative settingTable 5 lists the perioperative complications associated with endoscopic endonasal pituitary surgery. Complications are rare and usually due to lesion of the surrounding structures. Detection of the internal carotid artery with Doppler can prevent injury. Postoperative seizures may occur but the incidence is low (1%).33
Perioperative complications associated with endoscopic endonasal pituitary surgery.
| Koutourousiou et al.45 | Jakimovski et al.46 | De Witte et al.47 | |
|---|---|---|---|
| Intraoperative complications | |||
| Bleeding | – | – | – |
| Postoperative complications | |||
| Endocrine complications | |||
| Diabetes insipidus | 9.6% | – | 4 (4.9%) |
| Pituitary insufficiency | 16.7% | – | 16 (19.8%) |
| CSF leak | 7.4% | 3% | 5 (6%) |
| Vision worsening | 2 (3.7%) | – | – |
| Meningitis | – | – | 1 (1.2%) |
| Number of patients | 54 | 203 | 83 |
Postoperative care after pituitary surgery requires careful airway management, and close neurologic and endocrine monitoring. Approximately 25% of patients develop postoperative transient diabetes insipidus lasting for days to weeks, and in 0.5% it is permanent. SIADH is reported in 9–25% and is usually manifests a week after surgery.48 CSF leak is the most frequent postoperative complication.
The endoscopic approach has less morbidity than a craniotomy and its use is increasing; e.g. endonasal endoscopic surgery has been reported for clipping of a ruptured vertebral-posterior inferior cerebellar artery aneurysm.49
Patients undergoing skull base surgery require post-operative intensive monitoring. Patients scheduled for pituitary surgery at high risk (above 60 years old, severe comorbidities, clinically active acromegaly, Cushing's disease, large tumor with risk of hypothalamic dysfunction or intraoperative or early postoperative complications), may require “overnight intensive care”.50
FundingNone.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fàbregas N, Hurtado P, Gracia I, Craen R. Anestesia para Neurocirugía Mínimamente Invasiva. Rev Colomb Anestesiol. 2015;43:15–21.