Objective: To evaluate the effectiveness and safety of oxygen supplementation (inspired fraction of oxygen, FiO2) in high concentrations versus low concentrations, given with the aim of reducing complications in patients undergoing surgical procedures under general anesthesia.
Methods: A systematic review and a meta-analysis were performed following the methodology proposed by the Cochrane Collaboration.The review included controlled clinical trials conducted in patients undergoing surgical procedures under general anesthesia. After conducting data base searches (PUBMED, CENTRAL y LILACS), and once the relevant studies were identified, additional snowballing ambispective and grey literature searches were done.
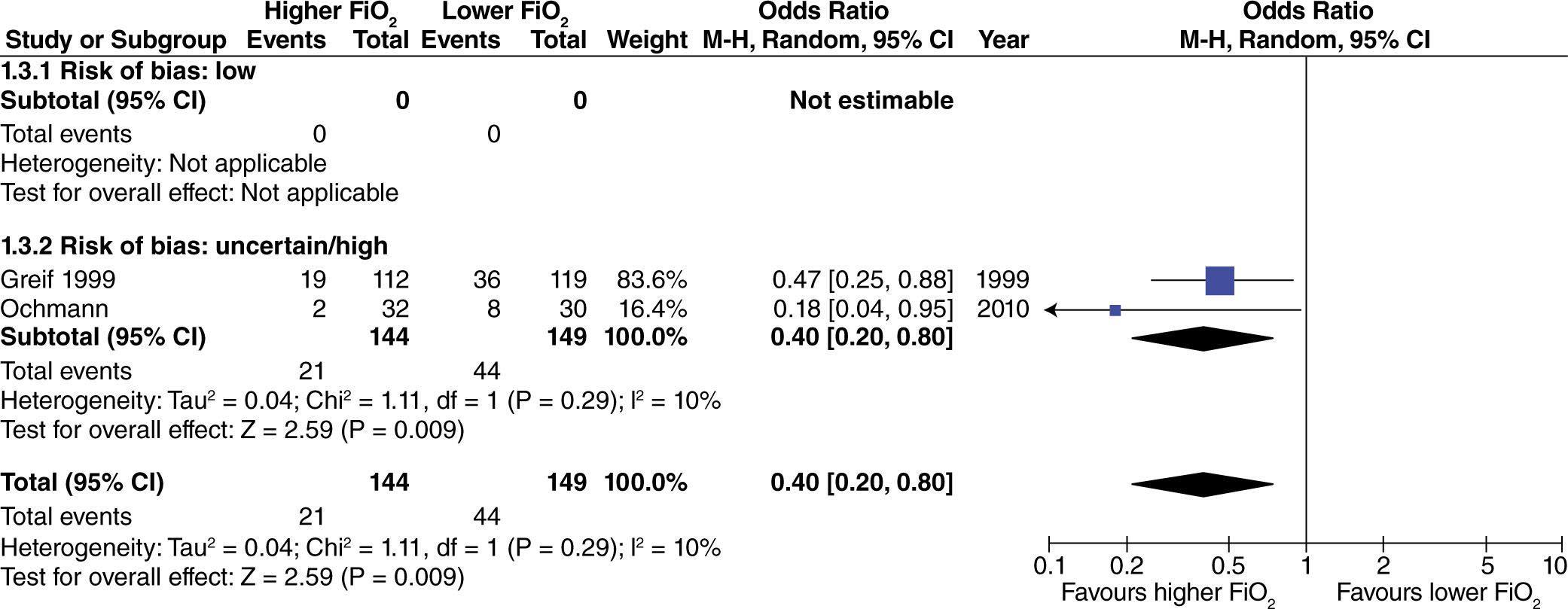
Results: Of the 17 clinical trials finally included (4844 patients), 7 were considered to a have a low risk of bias. High FiO2 levels reduce post-operative nausea and vomiting only in surgeries with extensive intestinal manipulation (odds ratio [OR] 0.40; 95% confidence interval [CI] , 0.20 to 0.80). In this same clinical setting, the risk of surgical site infection (OR 0.46; 95% CI, 0.29 to 0.74), and mortality (OR 0.17; 95% CI, 0.03 to 0.99) are also reduced. There was no impact on the need for rescue anti-emetic administration, length of stay in the post-anesthetic care unit, unexpected admission to the intensive care unit, or post-operative hospital stay in any of the surgical populations.
Conclusions: Intra-operative oxygen supplementation in high concentrations (≥ 60%) might reduce the risk of surgical site infection and mortality in surgeries with extensive intestinal manipulation
© 2012 Sociedad Colombiana de Anestesiología y Reanimación. Published by Elsevier. All rights reserved.
Objetivo: Evaluar la efectividad y la seguridad del suministro de oxígeno (fracción inspirada de oxígeno, FiO2) en concentraciones altas comparado con concentraciones bajas, para poder disminuir complicaciones en pacientes sometidos a procedimientos quirúrgicos bajo anestesia general.
Métodos: Se realizaron una revisión sistemática y un meta-análisis siguiendo la metodología propuesta por la Colaboración Cochrane. Se incluyeron experimentos clínicos controlados llevados a cabo en pacientes adultos sometidos a procedimientos quirúrgicos bajo anestesia general. Se hizo una búsqueda en bases de datos (PUBMED, CENTRAL y LILACS) y, con los estudios pertinentes identificados, se complementó con una nueva búsqueda ambispectiva en bola de nieve y en fuentes de literatura gris.
Resultados: Se incluyeron 17 experimentos clínicos (4.844 pacientes), de los cuales siete fueron considerados de bajo riesgo de sesgo. Las FiO2 altas disminuyen la náusea y el vómito posoperatorio solo en cirugías de manipulación intestinal extensa (odds ratio [OR] 0,40; intervalo de confianza [IC] 95%, 0,20 a 0,80). En este mismo escenario clínico, también disminuyen el riesgo de infección del sitio operatorio (OR 0,46; IC 95%, 0,29 a 0,74) y la mortalidad (OR 0,17; IC 95%, 0,03 a 0,99). La necesidad de antiemético de rescate, tiempo de estancia en la unidad de cuidado postanestésico, admisión no esperada a la unidad de cuidados intensivos y tiempo de estancia hospitalaria posoperatoria no se afectan en ningún tipo de población quirúrgica.
Conclusiones: El oxígeno suplementario intraoperatorio en concentraciones altas (≥ 60%) podría disminuir el riesgo de infección del sitio operatorio y la mortalidad en cirugías en las que se produce manipulación intestinal extensa
© 2012 Sociedad Colombiana de Anestesiología y Reanimación. Publicado por Elsevier. Todos los derechos resevados.
Oxygen (O2) is given routinely to all patients in anesthetic procedures, but concentrations vary between 30% and 100% in all cases.
Over the past two decades, several experimental trials assessing the possibility that the administration of high inspired fractions of oxygen (FiO2) might influence outcomes after certain types of surgeries have been published.1
Post-operative nausea and vomiting (PONV) are among the most undesirable side effects of general anesthesia, and they may even produce more discomfort than post-operative pain itself.2 On the other hand, the pathophysiology of PONV is still not fully understood.3
The idea of the anti-emetic properties of O2 came about as a result of the effect observed when the use of nitrous oxide(N2O) is reduced or avoided altogether.4 Some experimental studies have reported the anti-emetic effect of increasing the FiO2 in patients undergoing abdominal surgery.5,6 Moreover, some authors have proposed that oxygen administration in high concentrations may reduce intestinal hypoxia (resulting from surgical stress) and, consequently, reduce serotonin release produced because of its local vasodilatory effect.7
Surgical site infection (SSI) is a frequent and often severe complication that occurs after a surgical procedure.8 It is essential to optimize perioperative conditions, with the first few hours after bacterial contamination being critical for the establishment of wound infection.9 Partial oxygen pressure in the surgical wound is usually low at the end of the procedure, increasing the risk of infection because the eradication of the bacterial inoculum is dependent on the oxidation capacity of neutrophils.10,11 Hence the proposed idea of reducing the incidence of SSI by increasing intraoperative ¿oxygen?12–15 However, previous systematic reviews have not considered the influence on outcomes of the risk of bias of individual studies, or of intestinal manipulation.
The objective of this systematic review was to assess the effectiveness and safety of administering high oxygen concentrations (≥ 60%) compared to low concentrations (≤ 40%) as a way to reduce complications in patients undergoing surgical procedures under general anesthesia.
MethodsThis systematic review was made using the Cochrane Collaboration methodology,16 in accordance with the recommendations of the PRISMA Statement,17 and using the R-AMSTAR tool.18
Eligibility criteriaTypes of studiesOnly randomized controlled trials were included.
Types of participants and clinical scenariosThe studies included had been conducted in adult and pediatric patients undergoing surgical procedures under general anesthesia. Studies conducted in obstetric patients were excluded.
Types of interventionsThe experimental intervention was defined as the administration of intra-operative supplemental oxygen in high concentrations (≥ 60%). The comparison was made with a control intervention consisting of the administration of low concentrations of oxygen (≤ 40%).
Types of outcomesThe following outcomes were evaluated in accordance with the definitions of each study:
- -
Overall PONV
- -
Rescue anti-emetic administration
- -
Length of stay in the Post-Anesthesia Care Unit (PACU)
- -
Surgical site infection
- -
Admission to the Intensive Care Unit (ICU)
- -
Length of Hospital stay
- -
Atelectasis
- -
Pneumonia
- -
Mortality
The following electronic databases were searched:
- -
MEDLINE (Ovid SP, from 1966 to date)
- -
Cochrane Central Register of Controlled Trials CENTRAL (The Cochrane Library, current issue).
- -
LILACS (BIREME interface, from 1982 to date).
Specific strategies were used for each database, developed on the basis of the strategy designed for MEDLINE:
(((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (clinical trials as topic[mesh:noexp]) OR (randomly[tiab]) OR (trial[ti])) NOT (animals[mh] NOT (humans[mh] AND animals[mh]))) AND ((perioperat* OR intraoperat*) AND oxygen[tiab])
Search in other sourcesBased on the relevant papers identified, new terms were obtained in order to enrich the proposed search strategies. A manual search was performed on the references listed in the selected publications, in order to identify additional studies in articles, conference proceedings and abstracts. The snowballing search strategy was used based on the relevant publications using "related articles" in PubMed and "citing articles" in ISI Web of Science. Ongoing trials were identified:
Sources in the grey literature were also searched:
- -
Clinical Medicine Netprints Collection Index to Theses Canada Portal Networked Digital Library of Theses and Dissertations.
- -
New York Academy of Science Grey Source.
- -
Australian Digital Thesis Program Proquest
- -
Digital Theses ISTP on Web of Science British Library INSIDE (www.bl.uk/insideSIGLE)
- -
- -
- -
- -
- -
- -
Finally, the authors of relevant publications and listed pharmaceutical companies were contacted in order to identify additional published and non-published trials. No language or publication date restrictions were applied.
Data collection and analysisIdentification and selection of the studiesThe authors identified the titles and abstracts found as a result of the various searches – electronic, manual, snowballing, ongoing studies, grey literature, and contacts with the experts and the industry. All titles and abstracts were classified as relevant, irrelevant or uncertain. Full texts were selected for articles classified as relevant or uncertain by at least one of the authors.
The authors then used a checklist to make an independent selection of those identified publications that met the selection criteria. The statistical kappa was calculated in order to quantify consistency between reviewers. The titles were not masked, considering that the two reviewers are anesthesiologists and could recognize the source very easily, even if it was not stated. Inconsistencies were resolved by agreement.
Data extraction and handlingUsing a specific format, one of the authors (DARV) conducted an initial extraction of data on descriptive aspects related to the methods, participants, and interventions of each study. Then, both authors extracted the outcome data for the interventions independently and recorded them in a specific format. Inconsistencies were resolved through agreement. Data input into de RevMan was simple. Neither the authors nor the sources of publication were masked.
Assessment of the risk of bias in the studies includedBoth authors assessed the risk of bias in each of the studies independently, and recorded it in a specific format. The following aspects were assessed in accordance with the Cochrane Handbook for Reviews of Interventions (16):
- -
Generation of the assignment sequence
- -
Masking of the assignment
- -
Masking of the participants (patients, caregivers, outcomes reviewer, etc.)
- -
Incomplete data in the outcomes analysis
- -
Selective reporting of outcomes
- -
Other sources of bias
When the data required were not available in the study reports, additional information was sought through e-mail contact with the principal author of the trial.
Measurement of the effect of treatmentThe odds ratio (OR) was calculated for outcome dichotomies. The appropriate scale (hours, days) was used for continuous outcomes. Ninety-five per cent confidence intervals (95% CI) were calculated for all measurements.
Unit of analysisEach randomized patient was taken as a unit of analysis. For the management studies with multiple treatment groups, the "unit of analysis error" was avoided by combining similar groups in order to make a single comparison.16
Management of lost dataWhen required, the authors of the studies included were contacted in order to recover lost data. When contact was possible, available data were collected, and when not, the lost data were estimated (for example, standard deviations were calculated from standard errors or confidence intervals). If, despite these efforts it was not possible to obtain lost data, the analysis was done including only the data available.
Evaluation of heterogeneityHeterogeneity and inconsistency were evaluated using four strategies: comparison ofmethods, participantsand interventions of the studies (methodological heterogeneity), comparison of the types of patients (clinical heterogeneity), visual assessment of the forest plot, and Chi2, Tau2 and I2 statistics.
There is statistical heterogeneity when the P value of the Chi2 statistic is less than 0.10 or the I2 test is greater than 50%. The P value is undervalued for detecting heterogeneity in order to avoid false negative results when only a few studies or those with small sample sizes are evaluated. The degree of inconsistency between the studies was also evaluated using the I2 statistic, where a value greater than 50% indicates the presence of significant inconsistency.16
Evaluation of reporting biasReporting bias was approached by means of a detailed evaluation of the study methodology. The publication bias was assessed using the funnel plot for each outcome assessed by 10 or more trials.16
Data synthesisThe results of the studies were combined quantitatively, according to the measured outcome, using the Cochrane Collaboration Review Manager (RevMan 5.0) statistical package. The quantitative outcomes analysis was done on the basis of the "intention to treat". However, the data were analyzed per protocol when it was not possible to obtain the necessary data. Mean differences for continuous measured outcomes were calculated with the same scale, and the estimates were grouped using a "random effects" model.19
Subgroup analysesSubgroup analyses were performed for all outcomes, and also according to intestinal manipulation, on the basis of the modification to the classification proposed by Disbrow:20
- -
Absent
- -
Limited
- -
Extensive
Sensitivity analyses were performed in order to explore the origin of the heterogeneity, and the effect of the bias risk (low vs. uncertain/high) and the concomitant use of nitrous oxide (N2O) on the results.
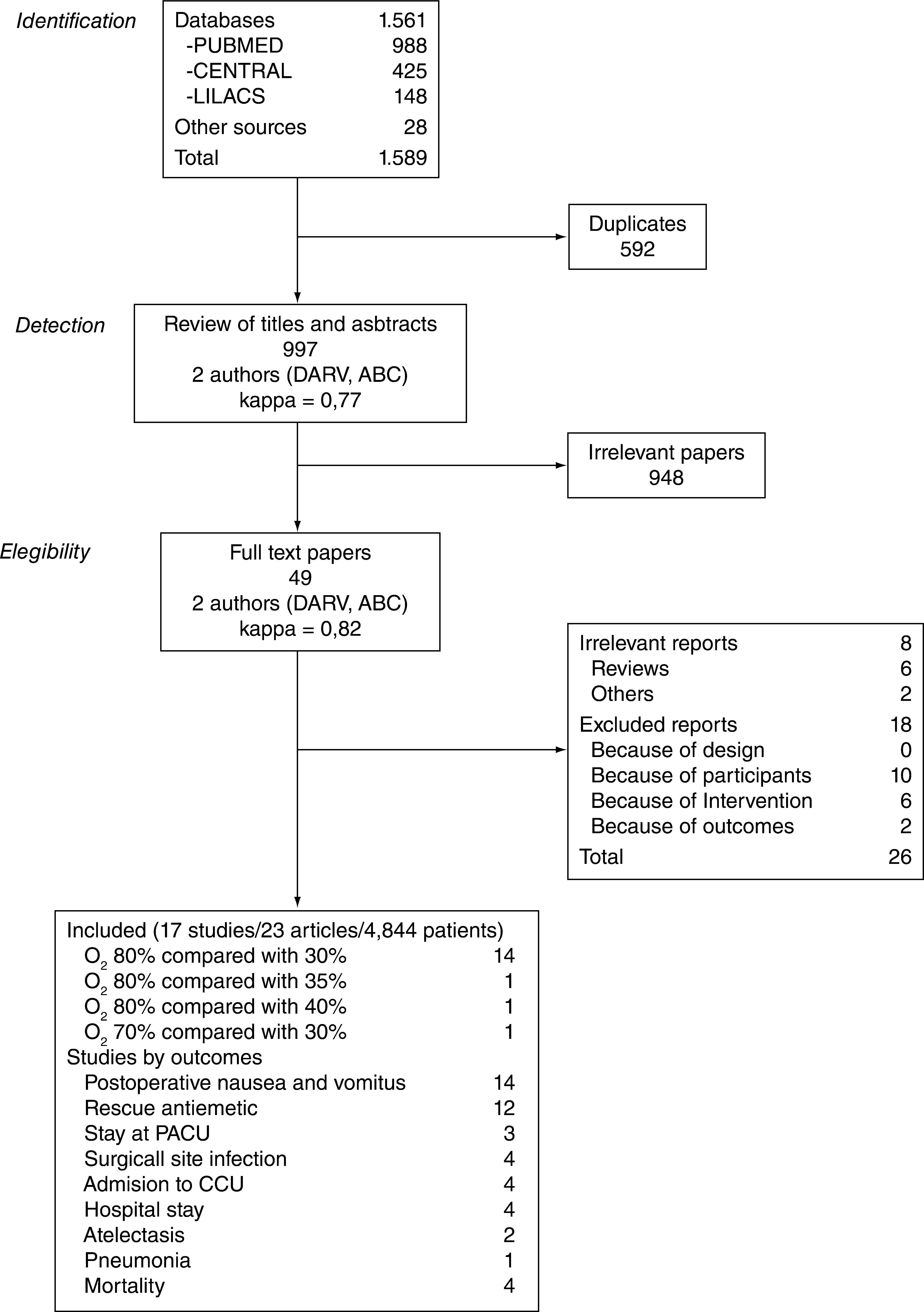
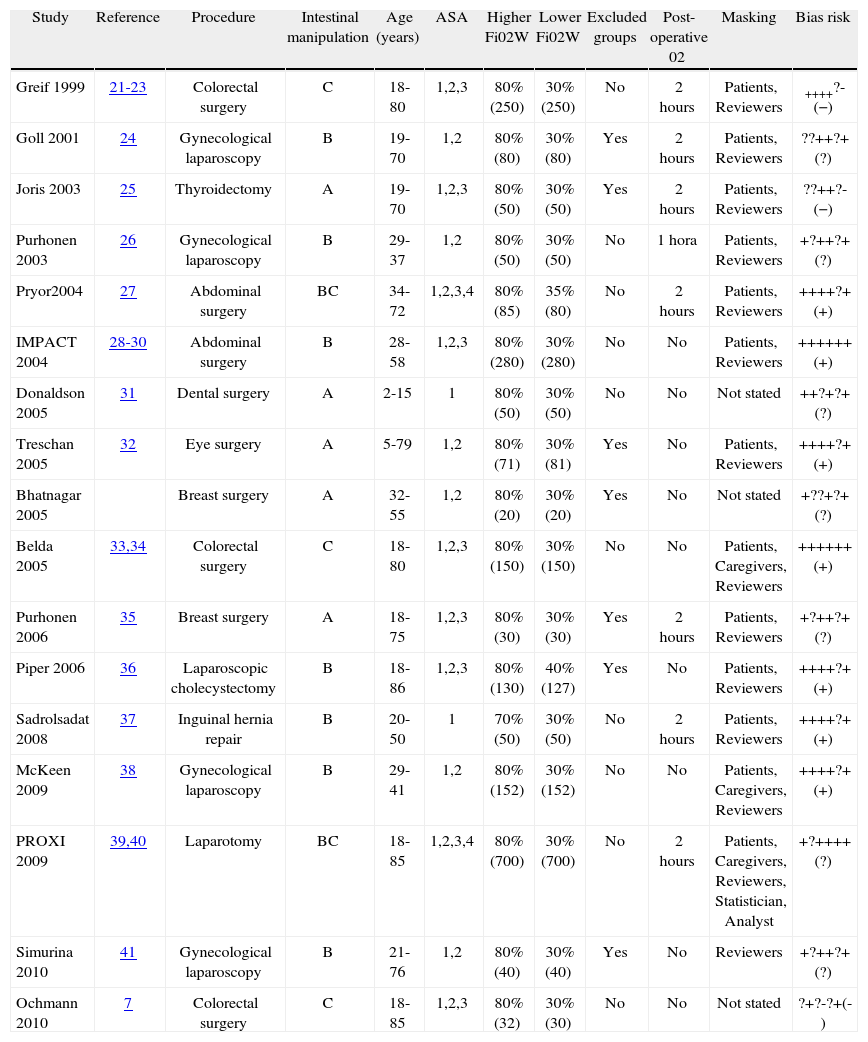
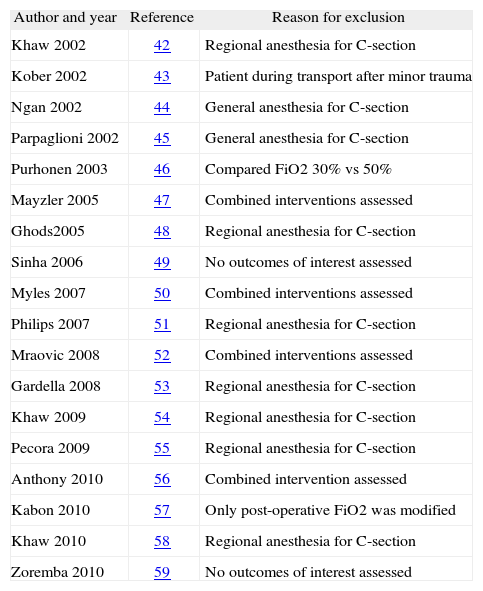
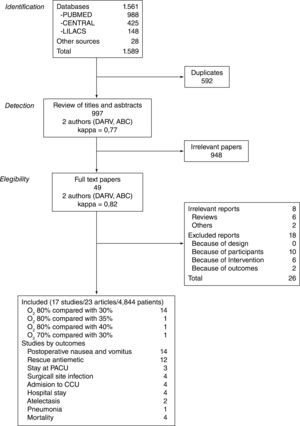
ResultsFigure 1 describes the process of selection of the studies. Tables 1 and 2 show the studies that were included and excluded, respectively. Of the 17 studies included, 7 (41%) were classified as having a "low risk of bias".
Included studies
| Study | Reference | Procedure | Intestinal manipulation | Age (years) | ASA | Higher Fi02W | Lower Fi02W | Excluded groups | Post-operative 02 | Masking | Bias risk |
| Greif 1999 | 21-23 | Colorectal surgery | C | 18-80 | 1,2,3 | 80% (250) | 30% (250) | No | 2 hours | Patients, Reviewers | ++++?-(−) |
| Goll 2001 | 24 | Gynecological laparoscopy | B | 19-70 | 1,2 | 80% (80) | 30% (80) | Yes | 2 hours | Patients, Reviewers | ??++?+(?) |
| Joris 2003 | 25 | Thyroidectomy | A | 19-70 | 1,2,3 | 80% (50) | 30% (50) | Yes | 2 hours | Patients, Reviewers | ??++?-(−) |
| Purhonen 2003 | 26 | Gynecological laparoscopy | B | 29-37 | 1,2 | 80% (50) | 30% (50) | No | 1 hora | Patients, Reviewers | +?++?+(?) |
| Pryor2004 | 27 | Abdominal surgery | BC | 34-72 | 1,2,3,4 | 80% (85) | 35% (80) | No | 2 hours | Patients, Reviewers | ++++?+(+) |
| IMPACT 2004 | 28-30 | Abdominal surgery | B | 28-58 | 1,2,3 | 80% (280) | 30% (280) | No | No | Patients, Reviewers | ++++++(+) |
| Donaldson 2005 | 31 | Dental surgery | A | 2-15 | 1 | 80% (50) | 30% (50) | No | No | Not stated | ++?+?+(?) |
| Treschan 2005 | 32 | Eye surgery | A | 5-79 | 1,2 | 80% (71) | 30% (81) | Yes | No | Patients, Reviewers | ++++?+(+) |
| Bhatnagar 2005 | Breast surgery | A | 32-55 | 1,2 | 80% (20) | 30% (20) | Yes | No | Not stated | +??+?+(?) | |
| Belda 2005 | 33,34 | Colorectal surgery | C | 18-80 | 1,2,3 | 80% (150) | 30% (150) | No | No | Patients, Caregivers, Reviewers | ++++++(+) |
| Purhonen 2006 | 35 | Breast surgery | A | 18-75 | 1,2,3 | 80% (30) | 30% (30) | Yes | 2 hours | Patients, Reviewers | +?++?+(?) |
| Piper 2006 | 36 | Laparoscopic cholecystectomy | B | 18-86 | 1,2,3 | 80% (130) | 40% (127) | Yes | No | Patients, Reviewers | ++++?+(+) |
| Sadrolsadat 2008 | 37 | Inguinal hernia repair | B | 20-50 | 1 | 70% (50) | 30% (50) | No | 2 hours | Patients, Reviewers | ++++?+(+) |
| McKeen 2009 | 38 | Gynecological laparoscopy | B | 29-41 | 1,2 | 80% (152) | 30% (152) | No | No | Patients, Caregivers, Reviewers | ++++?+(+) |
| PROXI 2009 | 39,40 | Laparotomy | BC | 18-85 | 1,2,3,4 | 80% (700) | 30% (700) | No | 2 hours | Patients, Caregivers, Reviewers, Statistician, Analyst | +?++++(?) |
| Simurina 2010 | 41 | Gynecological laparoscopy | B | 21-76 | 1,2 | 80% (40) | 30% (40) | Yes | No | Reviewers | +?++?+(?) |
| Ochmann 2010 | 7 | Colorectal surgery | C | 18-85 | 1,2,3 | 80% (32) | 30% (30) | No | No | Not stated | ?+?-?+(-) |
Intestinal manipulation classified as: A, without manipulation, B, limited handling, C, extensive manipulation. Items used in the risk of bias assessment16: Generation allocation sequence, allocation concealment, blinding of participants (patients, caregivers, outcome assessor, etc…), incompleteness in the outcomes analysis, selective reporting of outcomes, other sources of bias, (and evaluation).
Excluded studies
| Author and year | Reference | Reason for exclusion |
| Khaw 2002 | 42 | Regional anesthesia for C-section |
| Kober 2002 | 43 | Patient during transport after minor trauma |
| Ngan 2002 | 44 | General anesthesia for C-section |
| Parpaglioni 2002 | 45 | General anesthesia for C-section |
| Purhonen 2003 | 46 | Compared FiO2 30% vs 50% |
| Mayzler 2005 | 47 | Combined interventions assessed |
| Ghods2005 | 48 | Regional anesthesia for C-section |
| Sinha 2006 | 49 | No outcomes of interest assessed |
| Myles 2007 | 50 | Combined interventions assessed |
| Philips 2007 | 51 | Regional anesthesia for C-section |
| Mraovic 2008 | 52 | Combined interventions assessed |
| Gardella 2008 | 53 | Regional anesthesia for C-section |
| Khaw 2009 | 54 | Regional anesthesia for C-section |
| Pecora 2009 | 55 | Regional anesthesia for C-section |
| Anthony 2010 | 56 | Combined intervention assessed |
| Kabon 2010 | 57 | Only post-operative FiO2 was modified |
| Khaw 2010 | 58 | Regional anesthesia for C-section |
| Zoremba 2010 | 59 | No outcomes of interest assessed |
Intestinal manipulation classifiedas: A, absent manipulation; B, limited manipulation; C, extensive manipulation. Items used in assessing risk of bias:16 Generation of the assignment sequence, masking of the assignment, masking of participants (patients, caregivers, outcomes reviewer, etc.), incomplete data in the outcomes analysis, selective reporting of outcomes, other sources of bias (and final assessment).
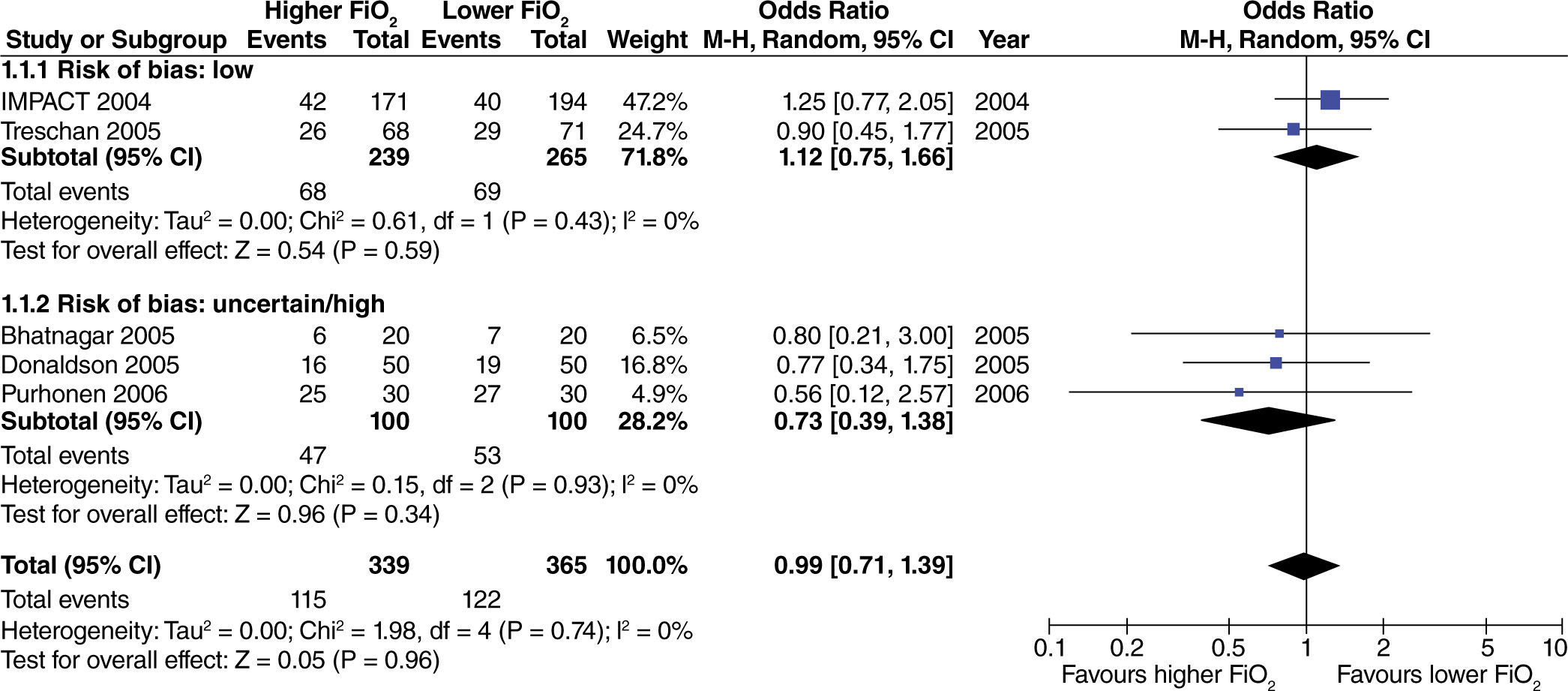
No significant differences were found regarding the incidence of post-operative nausea and vomiting (PONV) in surgeries where no intestinal manipulation was required. There were also no differences found between study results according to the risk of bias (fig. 2).
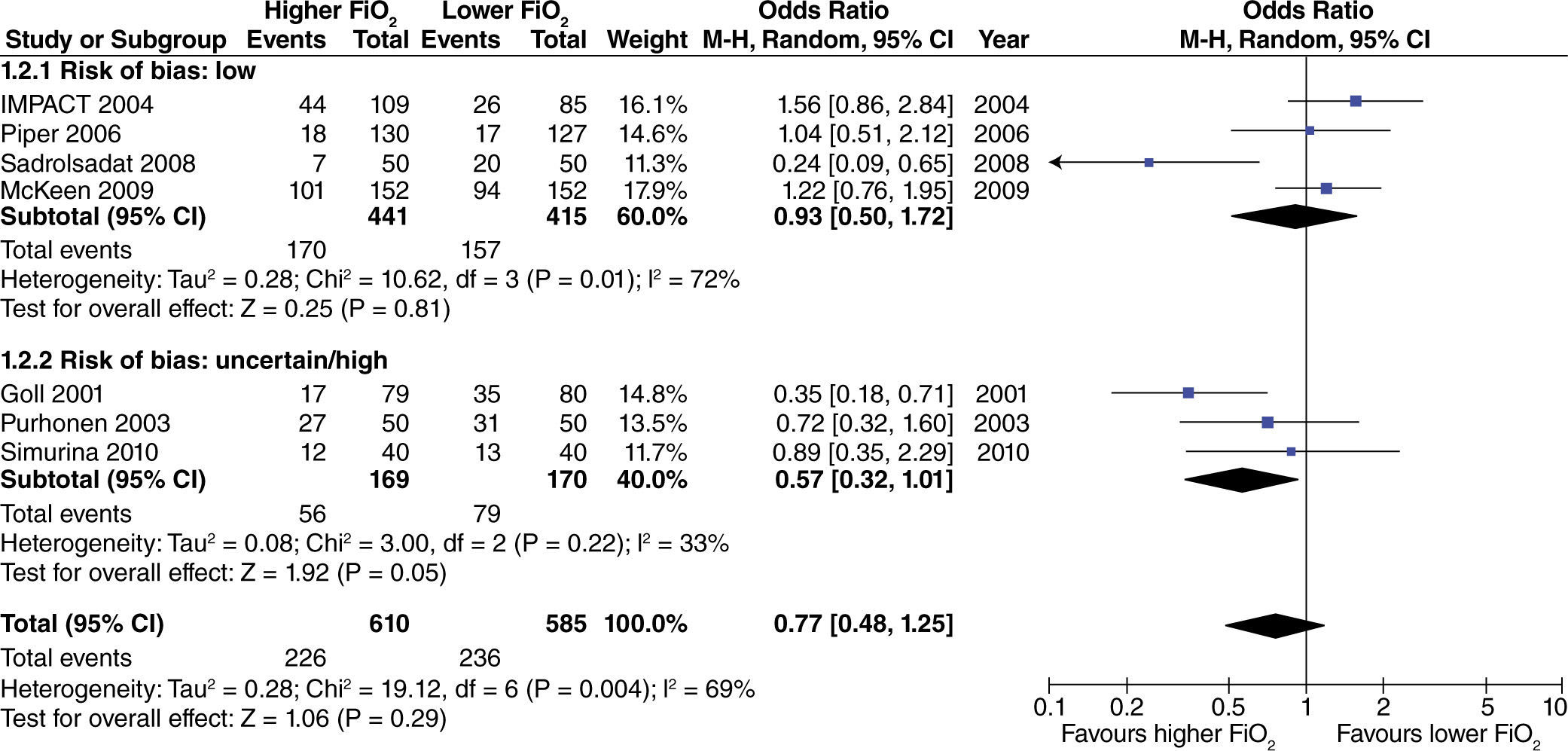
In studies including surgeries with limited intestinal manipulation, no differences were found in the incidence of PONV when high FiO2 was used. However, there was heterogeneity between studies, in particular among those with low risk of bias. This heterogeneity decreased (I2=0%) when the Sadrolsadat study37 -the only one where oxygen plus nitrous oxide was used-is excluded from the analysis. Likewise, there is no evidence of changes in the incidence of this outcome (fig. 3).
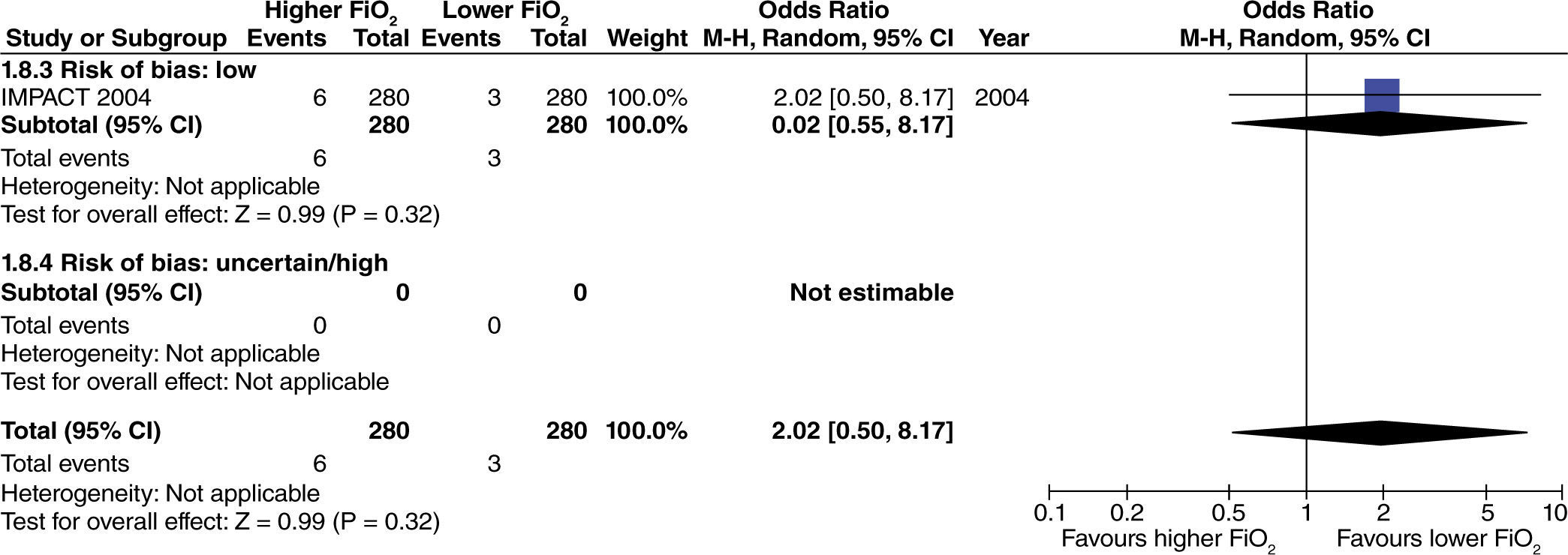
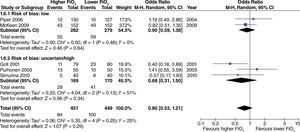
In surgeries with extensive intestinal manipulation, a beneficial effect was found with the use of high oxygen concentrations in reducing the incidence of PONV (fig. 4). This result was obtained only in studies with uncertain or high risk of bias.
In studies conducted with various types of surgeries where intestinal manipulation could not be classified, no differences were found regarding the incidence of PONV with the use of high FiO2. This result is independent from the risk of bias of the studies (fig. 5).
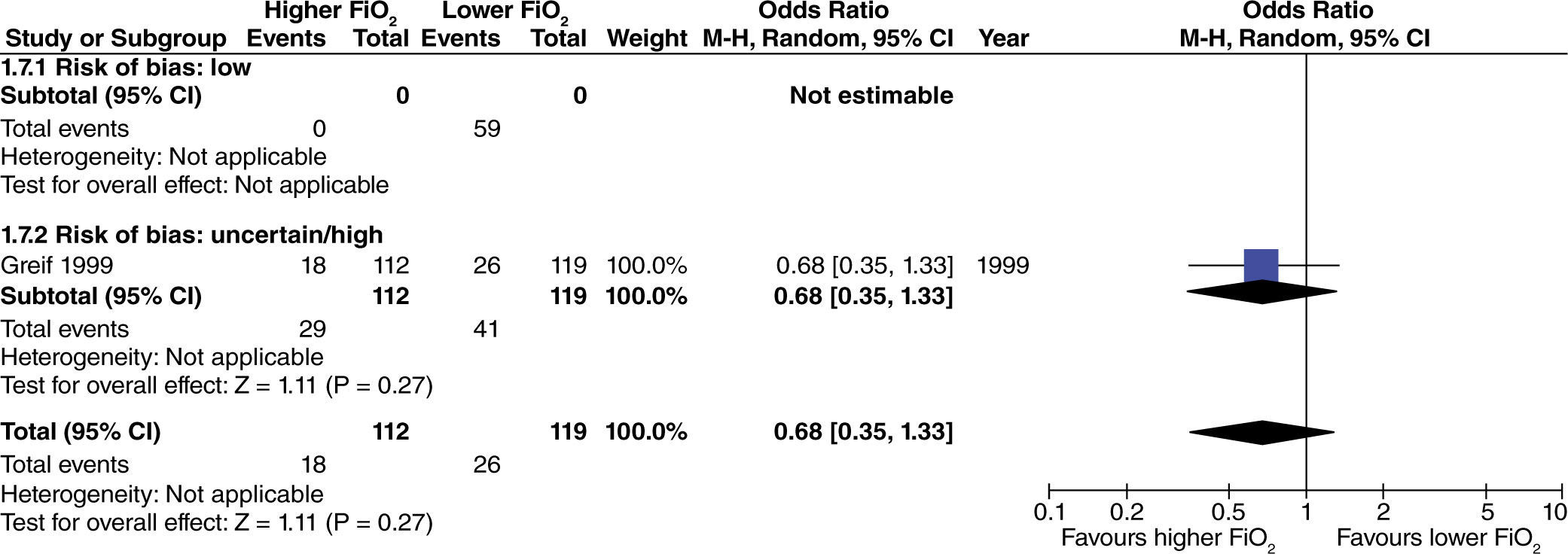
No differences were found regarding the need for rescue anti-emetic administration in surgeries with no intestinal manipulation (fig. 6). This result is consistent, despite differences in the risk of bias among studies.
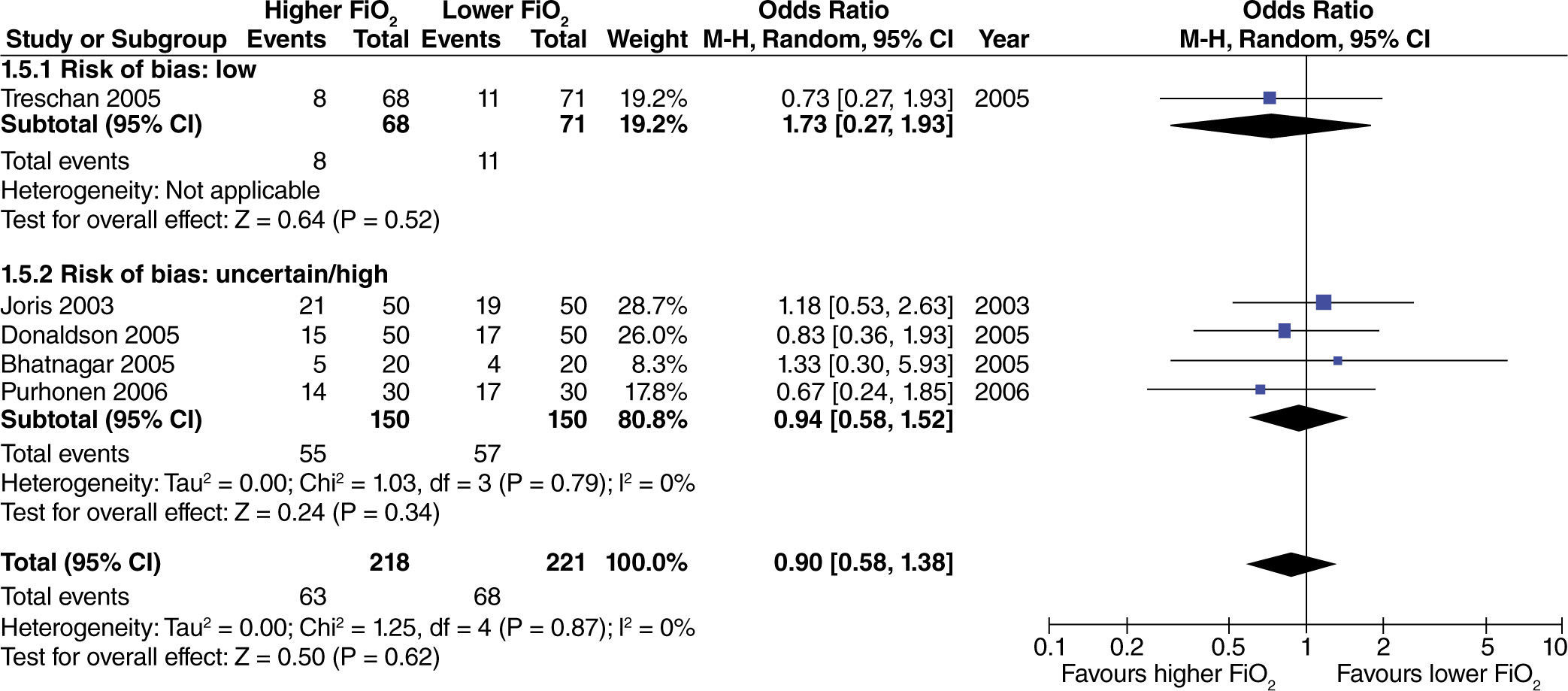
No differences were found regarding the need for rescue anti-emetic administration in surgeries with limited intestinal manipulation (fig. 7).
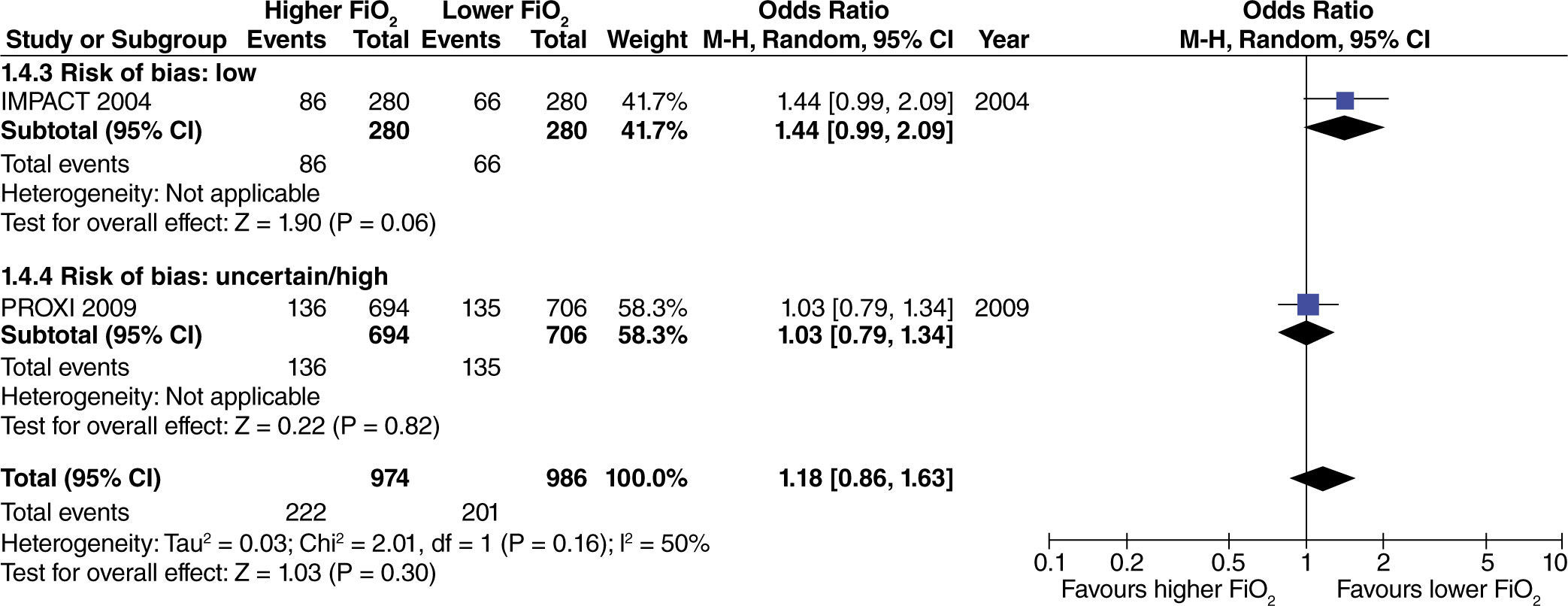
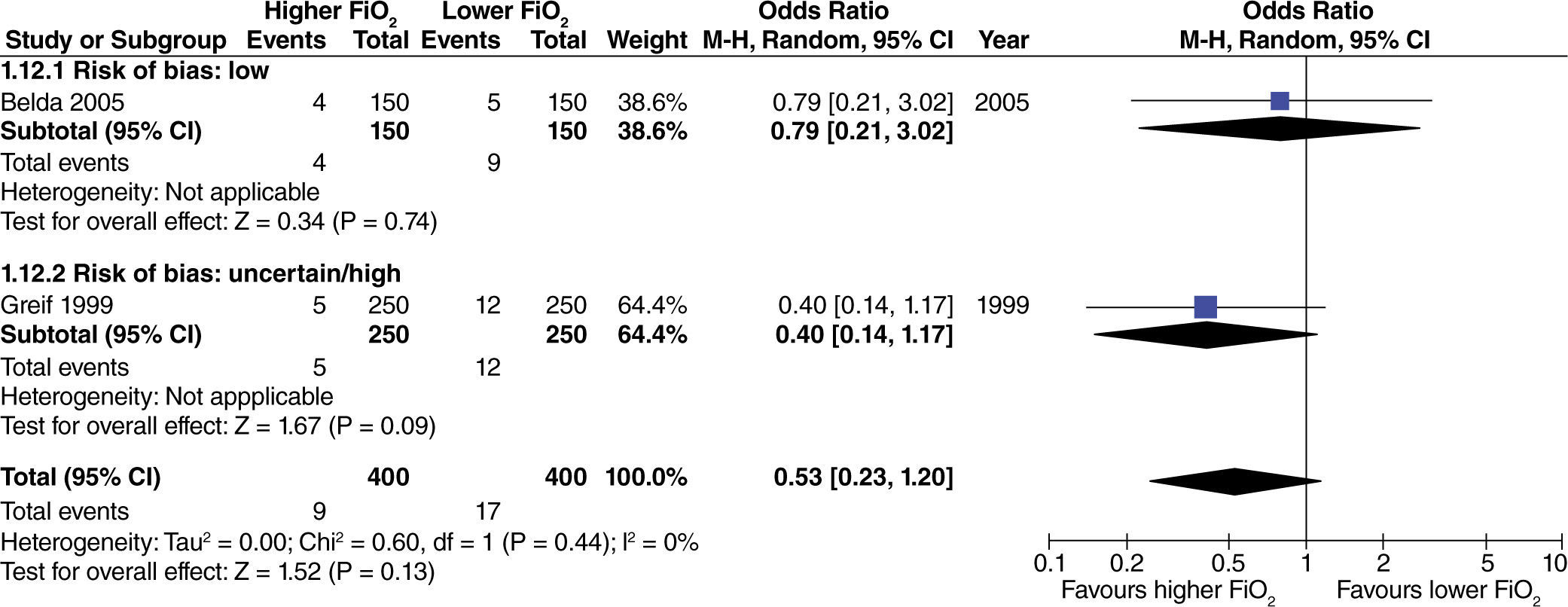
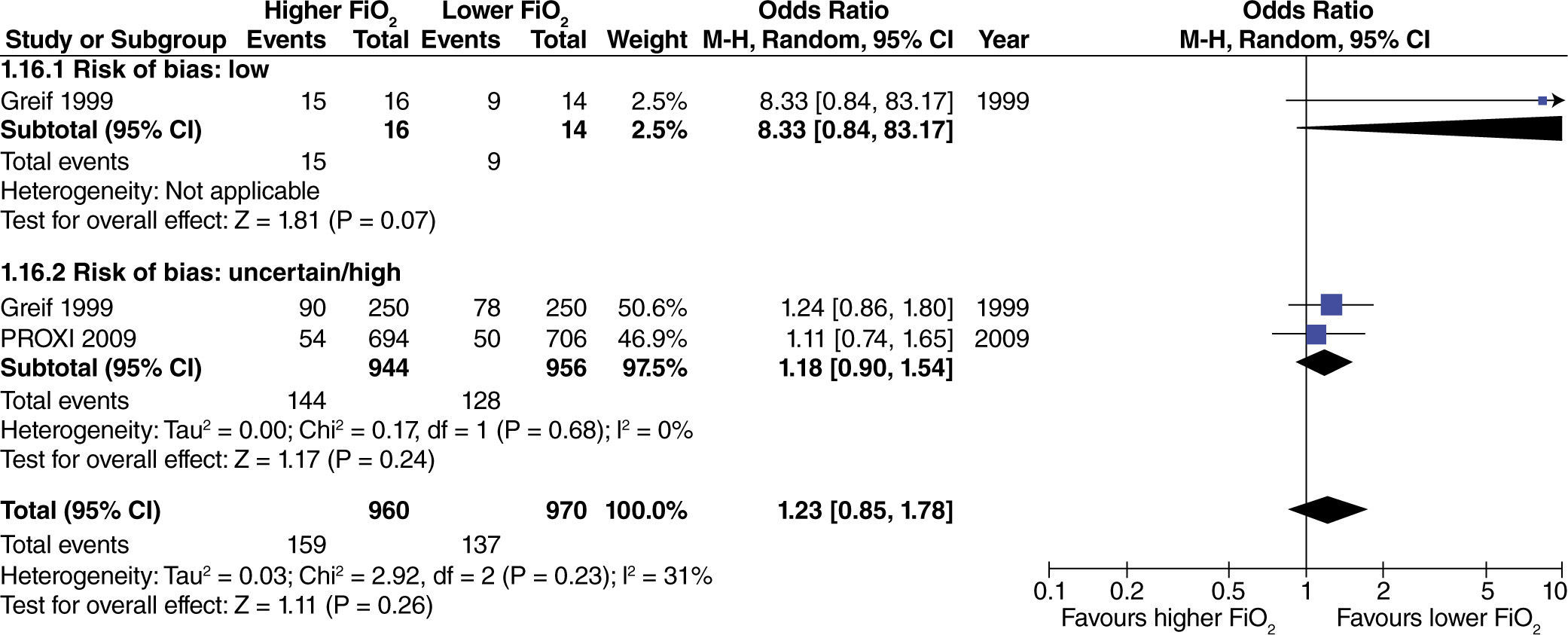
No differences were found regarding the need for rescue anti-emetic administration in surgeries with extensive intestinal manipulation (fig. 8). However, these data come from studies with high or uncertain risk of bias.
No differences were found in studies with heterogenous intestinal manipulation regarding the need for rescue anti-emetic administration with the use of high oxygen concentrations (fig. 9).
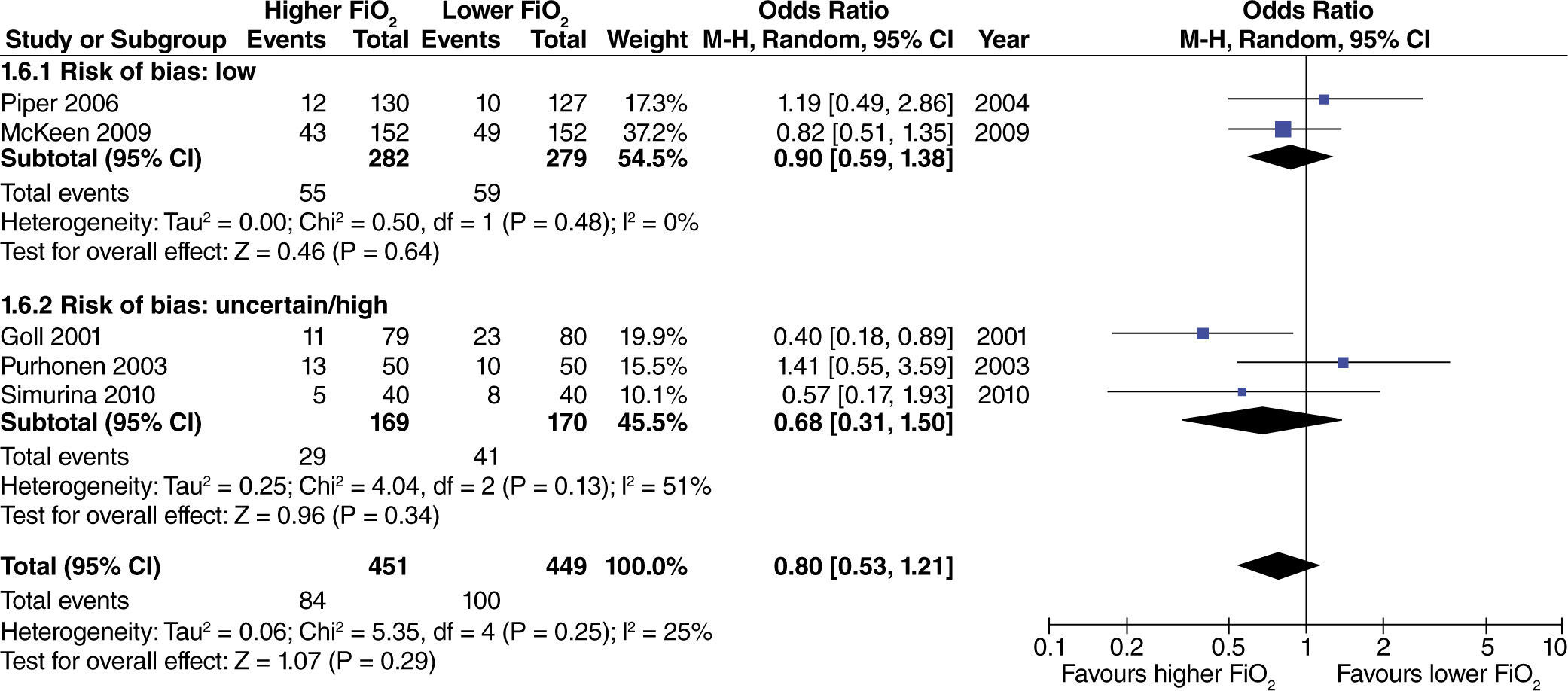
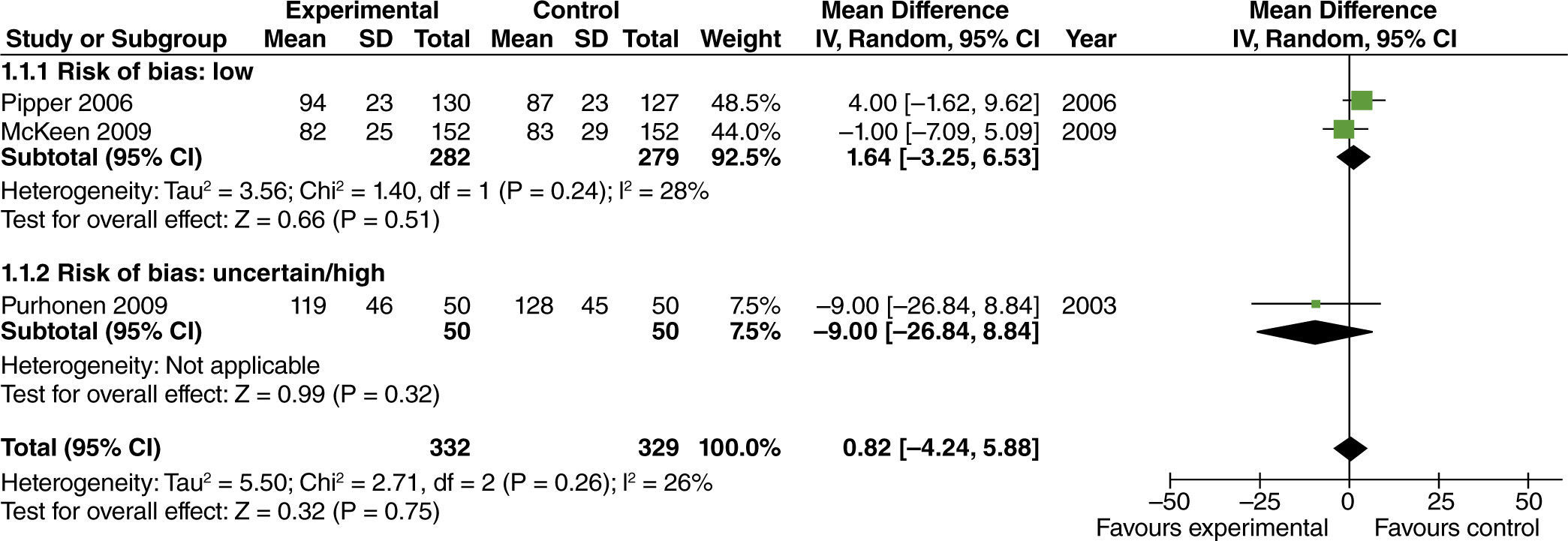
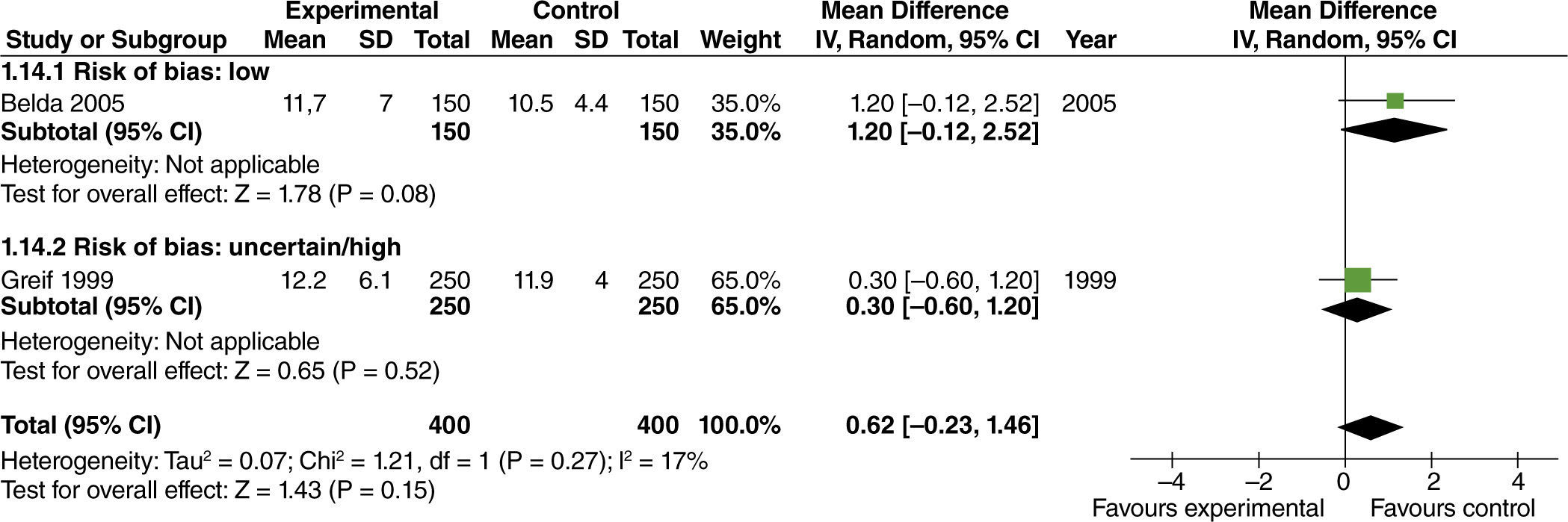
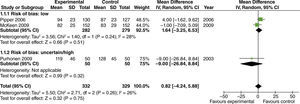
The effect of high FiO2 on the length of stay in the post-anesthetic care unit (PACU) was assessed in studies with limited intestinal manipulation that showed no evident difference (fig. 10).
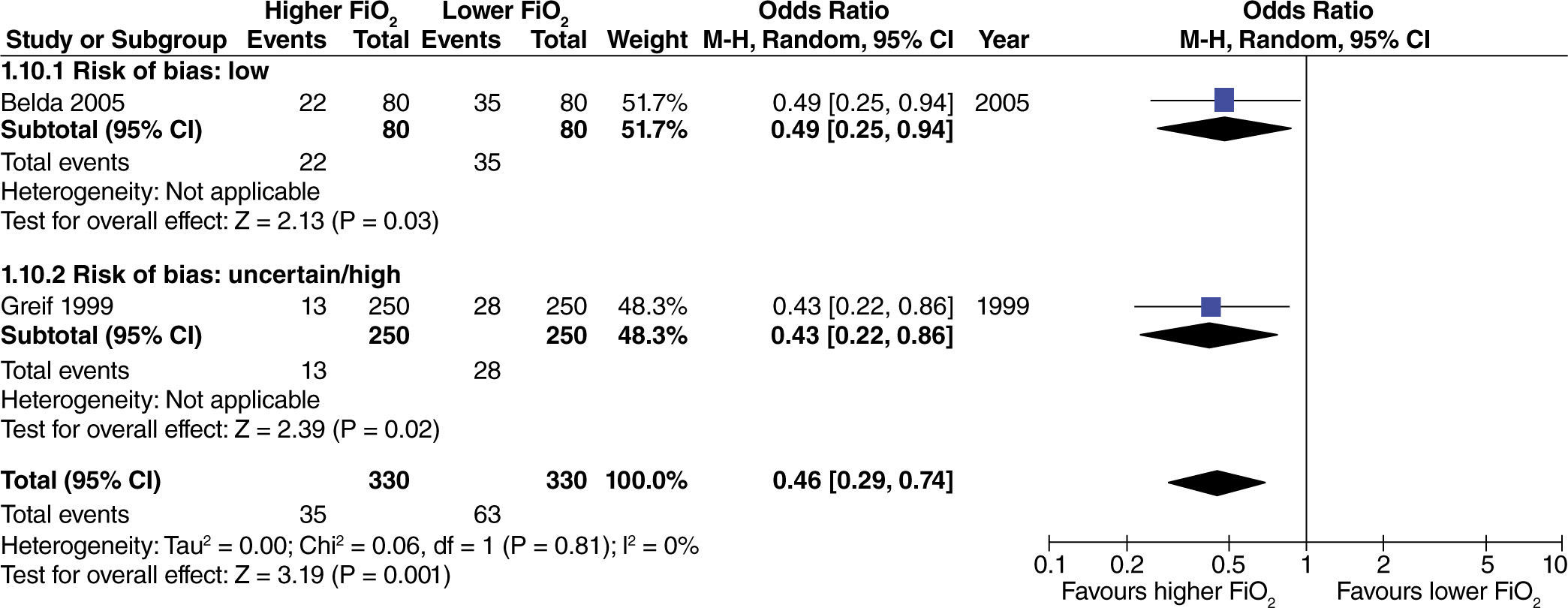
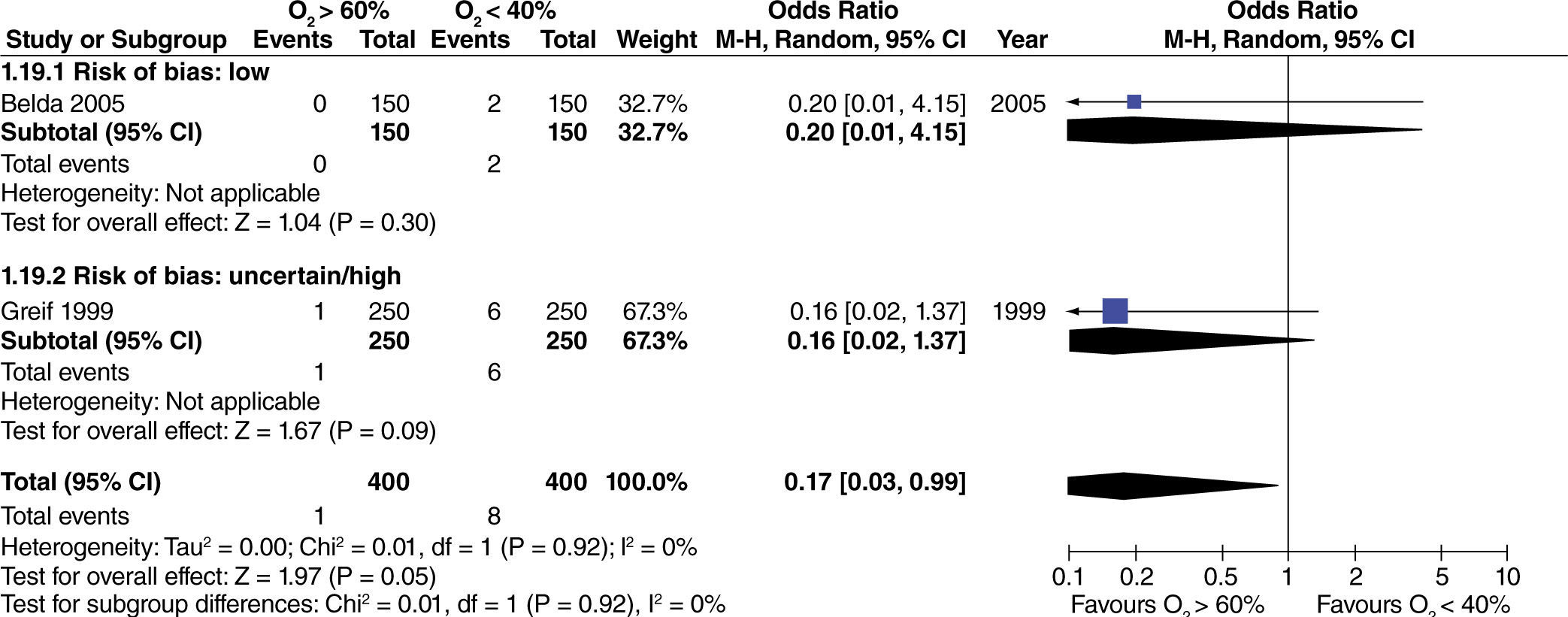
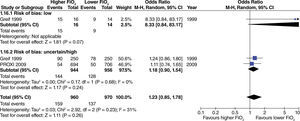
The effect of high oxygen concentrations on the incidence of surgical site infection (SSI) was evaluated in studies with extensive and heterogenous intestinal manipulation. A lower incidence of SSI was found in studies with extensive intestinal manipulation (fig. 11). There was no evidence that the risk of bias had an influence on this outcome.
In studies with heterogenous intestinal manipulation, differences were found only when the study by Pryor et al.27 was included, in which oxygen and nitrous oxide were used (fig. 12).
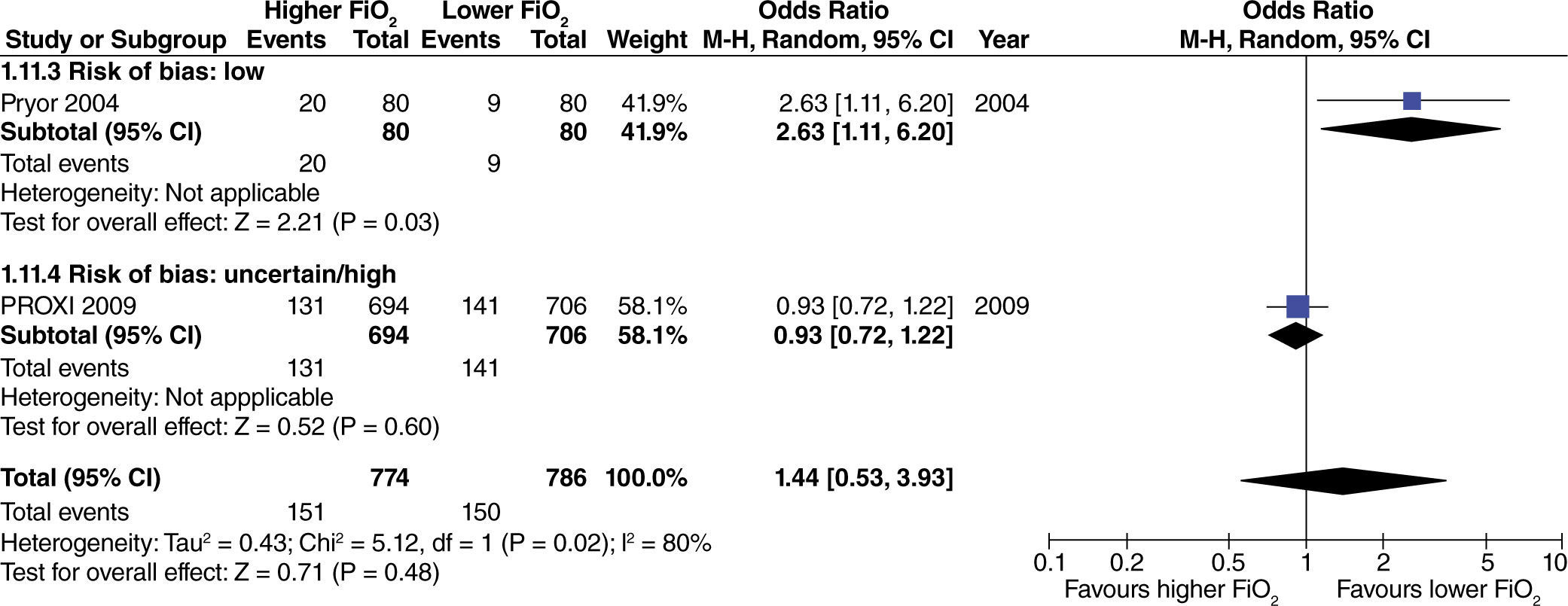
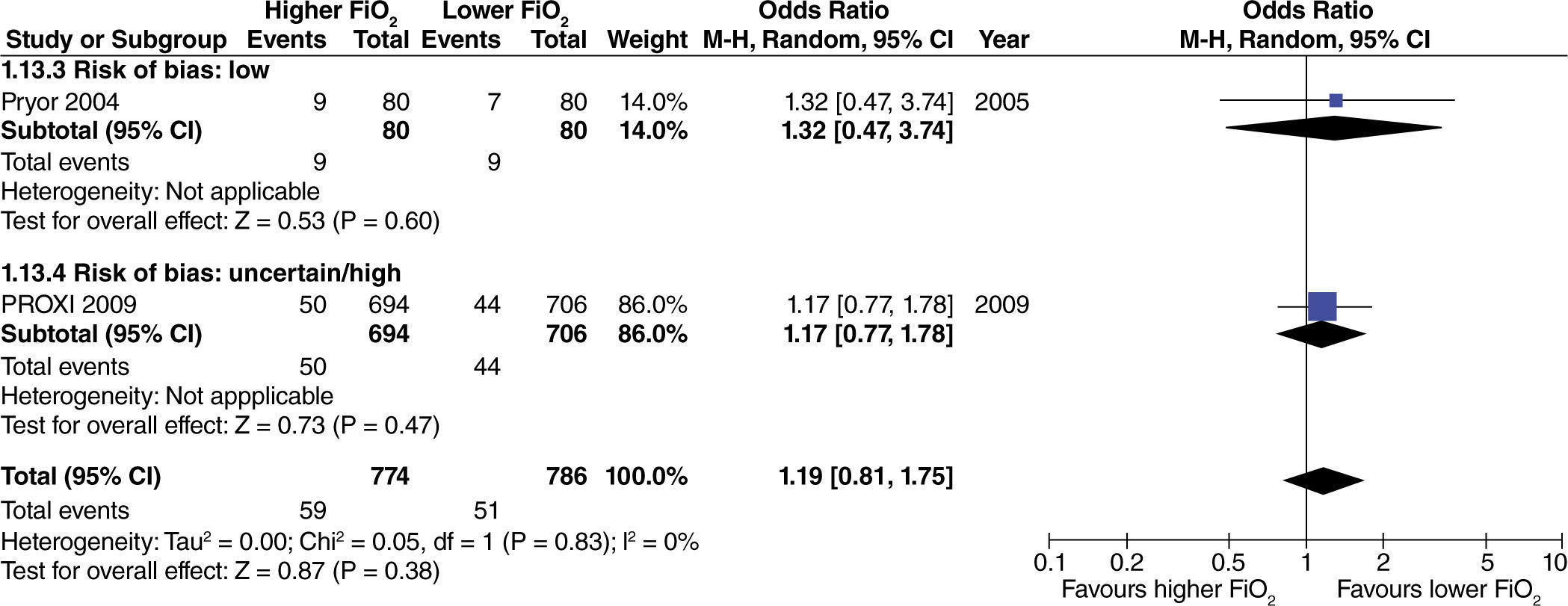
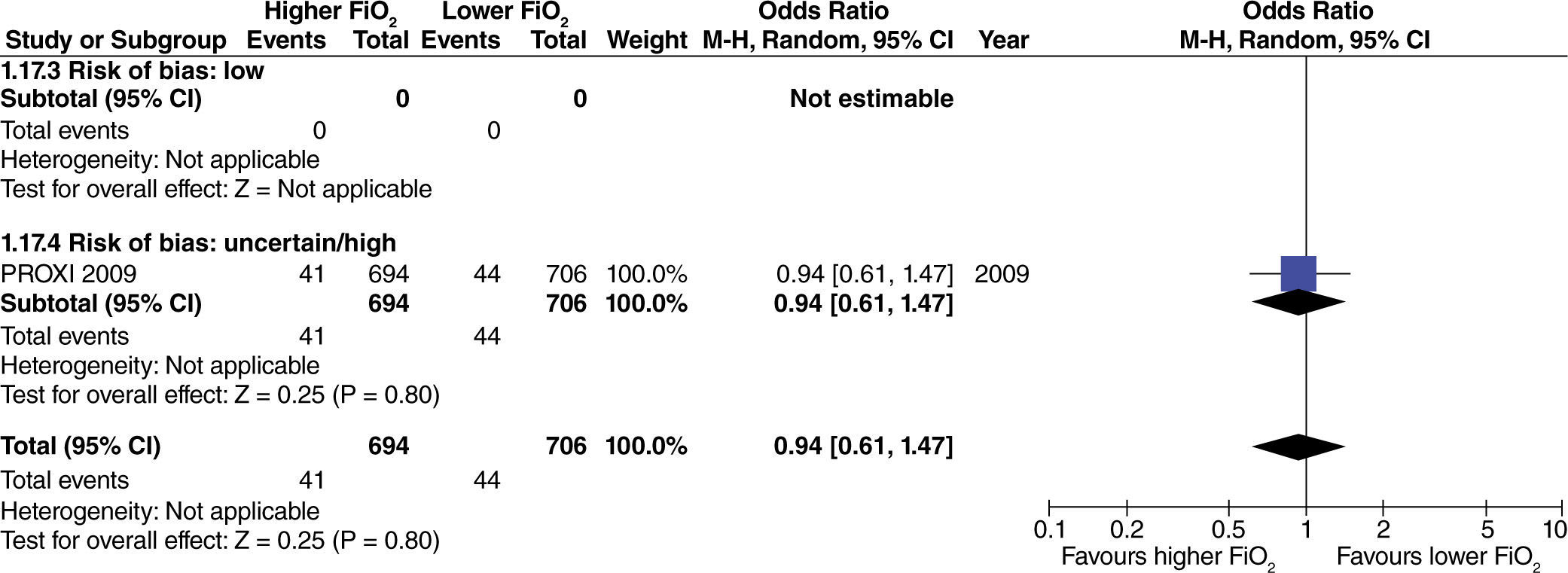
In studies with extensive intestinal manipulation, no effect was found with the use of high FiO2 on admission to the ICU (fig. 13). Neither was there any evidence of influence from the risk of bias on this outcome.
In studies with limited intestinal manipulation, no effect from high FiO2 was found on admission to the ICU (fig. 14) and neither was there any evidence of influence from the risk of bias on this outcome.
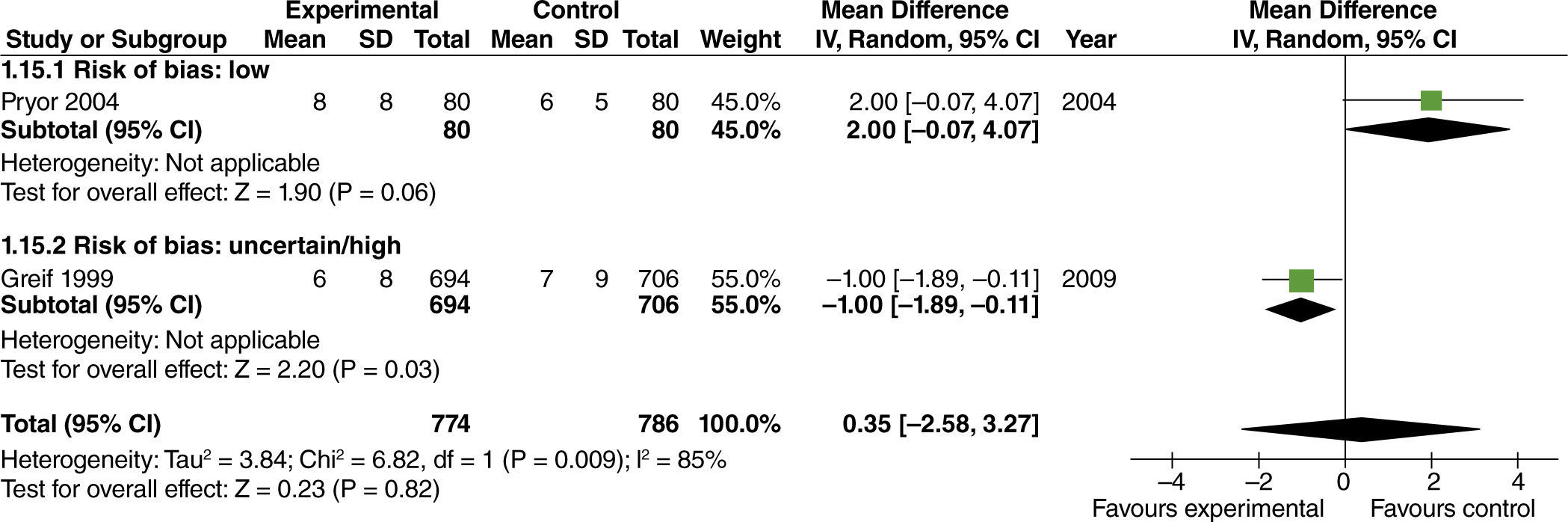
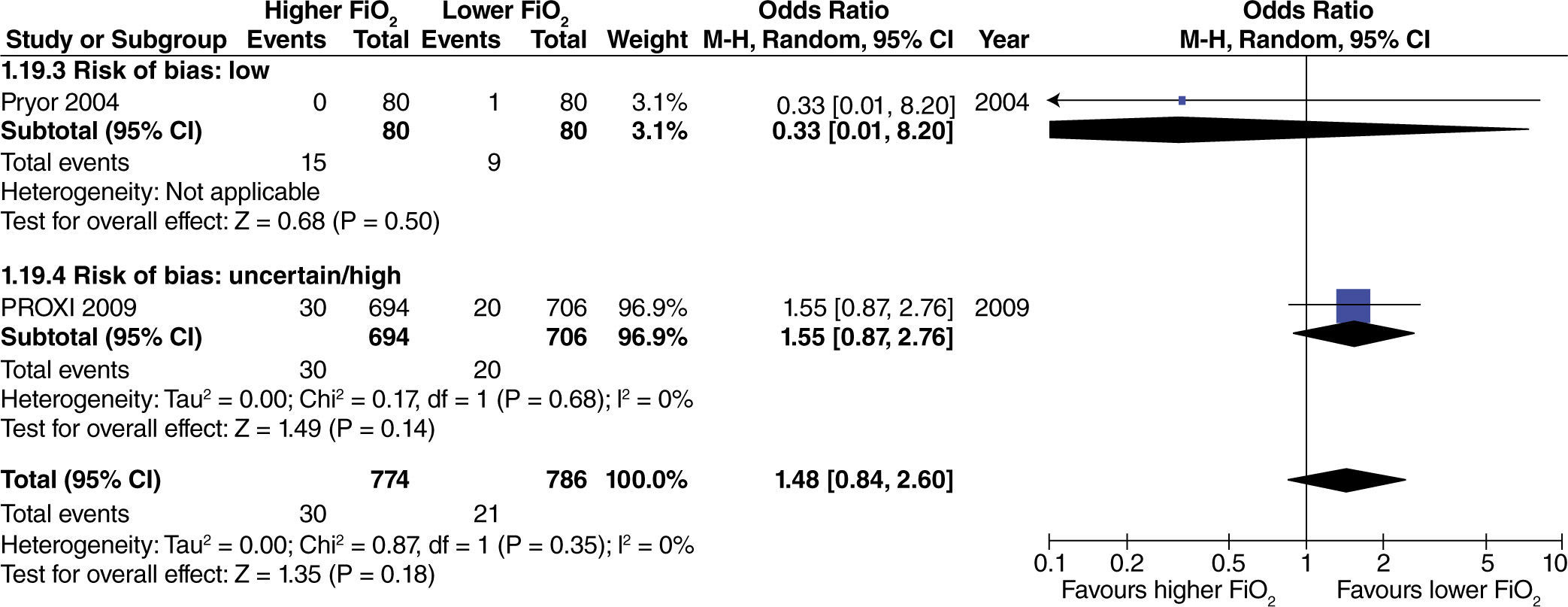
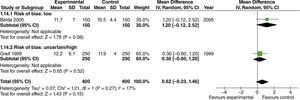
No effect from the administration of high oxygen concentrations on length of hospital stay was found in studies with extensive intestinal manipulation (fig. 15). There was no evidence of influence from the risk of bias on this result.
In studies with heterogenous intestinal manipulation, no reduced hospital stay was found with the use of high FiO2 (fig. 16). However, the influence of the risk of bias on this outcome was evident, considering that in the one study with a high risk of bias,40 hospital length of stay decreased by one day with the administration of high O2 concentrations.
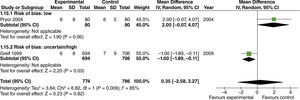
The use of different concentrations did not affect the incidence of atelectasis, regardless of the method used for diagnosis, the degree of intestinal manipulation, or the risk of bias of the studies (fig. 17).
No differences were found regarding the incidence of post-operative pneumonia with the use of high FiO2 during surgery (fig. 18).
The incidence of mortality was not affected with the use of high oxygen concentrations in studies with extensive intestinal manipulation and low risk of bias (fig. 19). Studies with a high risk of bias did not influence increased heterogeneity. When assessing the effect of high oxygen concentration on mortality without taking into consideration the risk of bias of the studies, a marginal reduction of this outcome was found (fig. 19).
The aforementioned result was not consistent when studies with heterogenous intestinal manipulation were taken into consideration (fig. 20), given the evident influence of the risk of bias on the results.
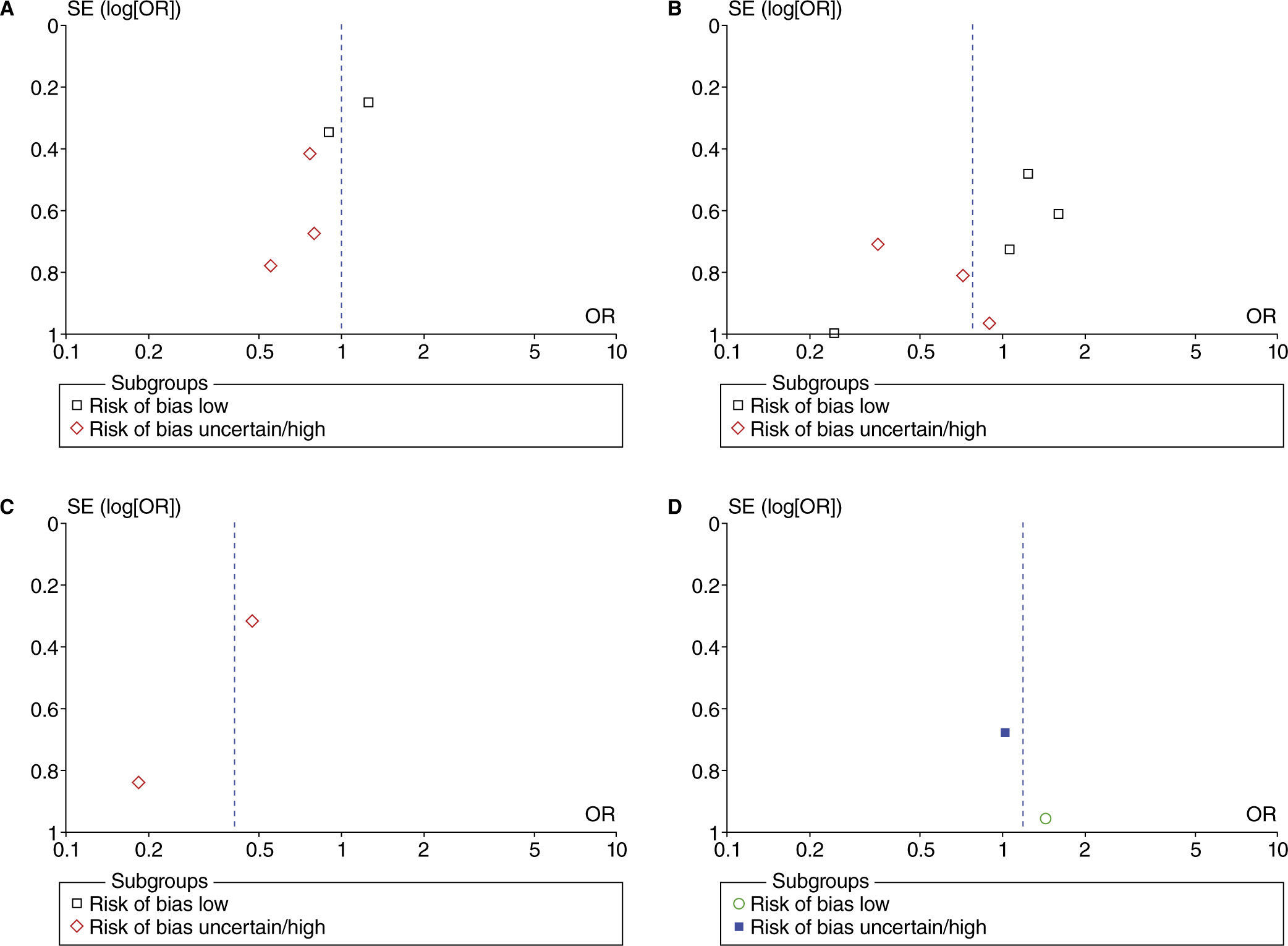
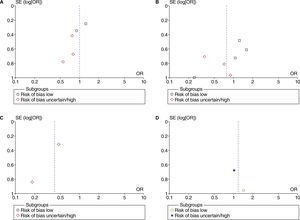
Funnel plot analyses did not show evidence of possible publication bias (fig. 21) in PONV. The publication bias was not assessed with funnel plots for other outcomes due to the small number of studies used for each meta-analysis.
Funnel plot of the effect of higher vs. lower FiO2 on post-operative nausea and vomiting. A, studies with no intestinal manipulation; B, studies with limited intestinal manipulation; C, studies with extensive intestinal manipulation; D, studies with heterogenous intestinal manipulation.
This systematic review found 17 relevant studies for answering the research question. It is important to mention that 10 out of 17 articles were classified as having high or uncertain risk of bias, reducing the amount of evidence with reliable results.
It was evident that the effect of different levels on the outcomes studied depended, in part, on the degree of intestinal manipulation to which the patients were subjected, although the trials included several surgical procedures that were not studied, such as major vascular surgery, where gut ischemia and manipulation are significant, as is the case with abdominal aortic dissection repair. It is important to highlight that in safety outcomes (atelectasis and pneumonia) no influence from the different levels was shown, although it is worth mentioning that a positive end-expiratory pressure (PEEP) of at least 5 mmHg was used in most of the studies.
Some systematic reviews with meta-analyses had already studied the effect of high FiO2 on several outcomes, in particular the reduction of PONV5,6 and SSI.12–15 However, none of them had considered systematically the effect of the methodological aspects of the individual studies over the final result. Moreover, they inappropriately combined studies where N2O or nitrogen was used as a second step, creating a confounding factor, with an influence on the results that needs to be studied. Indeed, this review found that the inclusion of such studies27,37 resulted in increased statistical heterogeneity, although it did not necessarily modify the results.
It is important to bear in mind that the subgroup analyses used in this meta-analysis diminishes the statistical power and accuracy of the results at the expense of reducing heterogeneity. For this reason, this review is subject to a high probability of false negative results, meaning that it might not find differences between the interventions, where they actually exist.
In conclusion, intra-operative supplemental oxygen at high FiO2 (≥ 60%) might reduce the risk of surgical site infection and mortality, exclusively in surgeries with extensive intestinal manipulation (e.g. colorectal surgery). Nearly 60% of the studies have an uncertain or high risk of bias, which makes it impossible to arrive at the irrefutable conclusion that high oxygen concentrations have antiemetic properties of clinical relevance. The need for rescue anti-emetic administration, the length of stay in the PACU, the unexpected admission to the ICU, or the length of post-operative hospital stay were not found to be affected in any of the surgical populations. FiO2 in the range used in the studies (30% to 80%) was also not found to have an effect on atelectasis or pneumonia, regardless of the degree of intestinal manipulation.
Additional research trials with low risk of bias are required in order to fill the knowledge gaps regarding the ideal concentration of intra-operative O2 in general anesthesia.
Competing InterestsSeveral of the studies included are attributed to OUTCOMES RESEARCH Consortium. The authors claim not having derived any financial benefit from the results of this systematic review. Received from OUTCOMES RESEARCH Consortium (www.or.org).
Funding sources: The authors' own resources