The purpose of this study was to demonstrate how a secondary school chemistry teacher in Hong Kong can evaluate his students’ attitudes toward chemistry lessons by means of the 12-item Attitude Toward Chemistry Lessons Scale (atcls). The teacher found that overall his students had a slightly positive attitude toward chemistry lessons in school, but the mean scores on two of the four subscales were lower than expected. He was interviewed to identify the underlying reasons. The information about student attitude toward chemistry lessons served as a catalyst to help the teacher to reflect on his curriculum planning and teaching effectiveness. The nature of the four subscales and the interview results are discussed.
Chemistry teacher educators worldwide have long been asking the question: How can we help chemistry teachers improve their curriculum planning and classroom teaching? One of the ways to enhance teacher professionalism is to develop their ability to conduct curriculum evaluation (Fistzpatrick, Sanders & Worthen, 2004; Preedy, 2001; Stufflebeam & Shinkfield, 2007). According to Preedy (2001), curriculum evaluation is the process of gathering evidence to make judgments about the value or worth of curriculum plans, processes and outcomes, as a basis for developing and improving them. Chemistry teachers can make their curricula and teaching more effective if they identify where they are weak and strong. The learning outcomes of school chemistry can be broadly classified into three domains: cognitive, psychomotor, and affective. The affective domain includes learning outcomes such as students’ attitudes, motivation, values, self-esteem, and self-efficacy. The importance of development of affective outcomes is well documented in the literature (see, for example, Anderson & Bourke, 2000; Popham, 2005), but many secondary school chemistry teachers do not know how to evaluate their students’ attitudes toward chemistry lessons. Even educational researchers are generally weak in this area of empirical research (Cheung, 2009a). Law (2002) has pointed out this problem succinctly: Although values, attitudes, and habits of mind are mentioned in many national science curriculum documents, they do not generally form part of formal assessment procedures. Nor has there been much research into how these attributes can be assessed. In consequence, they are unlikely to receive any significant attention in the teaching and learning process. (Law, 2002, p. 173)
Student attitudes are important dependent variables in the evaluation of science curricula (Bennett, Lubben, & Hogarth, 2007; Fraser, 1979; Gardner, 1975). Unfortunately, the literature on student attitude toward chemistry lessons is limited, as many previous studies focused mainly on attitudes toward science (Gardner, 1975; Osborne, Simon & Collins, 2003). Students may show different attitudes toward chemistry lessons and science because they are different attitude objects. To my knowledge, no study has been published where information about student attitude toward chemistry lessons was utilized to facilitate secondary school teachers to evaluate curriculum planning and classroom teaching. The purpose of this paper is to demonstrate how student attitudes can be evaluated by means of a questionnaire and utilized to help a teacher in Hong Kong to reflect on his chemistry teaching.
Formation and Measurement of AttitudesAttitude is a hypothetical construct used by social psychologists to understand and predict the behaviors of humans (Eagly & Chaiken, 1993). An attitude may be defined as a predisposition to respond in a favorable or unfavorable manner with respect to a given attitude object (Oskamp & Schultz, 2005). The attitude object can be anything, such as chemistry, chemists, chemistry lessons, topics taught in school chemistry, inquiry-based chemistry laboratory experiments, chemical education research, chemical weapons, and industrial chemistry. Thus, every student has many attitudes, on different aspects of chemistry. Chemistry educators should specify the attitude object clearly when conducting attitude research. The focus of this paper is on student attitude toward chemistry lessons taught in ordinary classrooms. The term ‘lessons’ refers to chemistry theory classes and laboratory classes in the secondary school (i.e., chemistry as experienced by students in school rather than out-of-school experiences obtained from external sources such as the media, museums, field trips, and friends).
It is noteworthy that an attitude is not behavior. To date, three major theoretical viewpoints have been used by social psychologists to conduct research on attitudes: the tri-component viewpoint, the separate entities viewpoint, and the latent process viewpoint (Cheung, 2009a; Oskamp & Schultz, 2005). The latent process viewpoint conceptualizes attitude as a latent variable that can be used as an explanation of the relationship between a stimulus and a person's behaviors. This viewpoint is better than the other two viewpoints because it is more consistent with the findings of contemporary attitude research.
How can an attitude toward chemistry lessons be formed? According to the latent process viewpoint, the chemistry lessons implemented in school can arouse a student's cognitive, affective, and/or behavioral processes (Oskamp & Schultz, 2005). These processes occur within the student and thus are not observable (see Figure 1). Together or separately, they can form an attitude toward chemistry lessons in school. In other words, psychologists have postulated that the chemistry lessons implemented in school can trigger some hidden processes within the student and an attitude is a general evaluative summary of the information derived from those hidden processes.
It is important to develop students’ positive attitudes to chemistry lessons in school due to two main reasons. Research has confirmed that attitudes are linked with academic achievement. For example, Salta and Tzougraki (2004) found that the correlation between high school students’ achievement in chemistry and their attitudes toward chemistry ranged from 0.24 to 0.41. Bennett, Rollnick, Green and White (2001) also found that undergraduate students who had a less positive attitude to chemistry almost invariably obtained lower examination marks. Another reason why it is important to develop students’ positive attitudes toward chemistry lessons is that attitudes predict behaviors (Glasman & Albarracín, 2006; Kelly, 1988). For example, Kelly (1988) reported that British students’ liking for a particular science subject was a good predictor of their actual choice of physics, chemistry, or biology in schools.
Obviously, the development of students’ positive attitudes regarding chemistry as a school subject is one of the major responsibilities of every secondary school chemistry teacher. But if attitude is an internal state, how can a chemistry teacher know that students possess a positive or negative attitude toward chemistry lessons? Because attitude is a latent variable, the existence of an attitude can only be inferred from some observable attitudinal responses (Eagly & Chaiken, 1998; Krosnick, Judd, & Wittenbrink, 2005). There are generally three classes of observable attitudinal responses: affective, cognitive, and behavioral (Oskamp & Schultz, 2005). The affective attitudinal responses are the feelings and emotions that a person has toward the attitude object. The cognitive attitudinal responses refer to a person's evaluative beliefs that she or he has about the attitude object. The behavioral attitudinal responses are not behaviors per se, but are the person's action tendencies toward the attitude object.
To quantify student attitudes toward chemistry lessons, I have developed an instrument for use with secondary school students based on these three classes of attitudinal responses (Cheung, 2009a). It is called Attitude Toward Chemistry Lessons Scale (atcls). The atcls was developed in several stages. The original scale consisted of 20 items with a Likert format. Students responded to the items on a 7-point rating scale with labels strongly disagree, moderately disagree, slightly disagree, not sure, slightly agree, moderately agree, and strongly agree. Reliability tests and confirmatory factor analysis resulted in 12 items forming four subscales (three item each). They were written in Chinese and have been translated into English for reader information (see Table 1). The first subscale focuses on the feelings a student has toward the chemistry theory lessons implemented in school, while the second subscale evaluates whether a student likes chemistry laboratory classes in school. Thus, the first and second subscales are concerned with affective attitudinal responses. The third subscale is cognitive in nature and refers to the evaluative beliefs that a student holds about the importance and usefulness of school chemistry. The fourth subscale is concerned with a student's behavioral tendencies to respond to school chemistry. Cheung (2009a) administered the 12-item atcls to a sample of 954 chemistry students in Hong Kong. The internal consistency reliability of the atcls was adequate with the Cronbach alpha values for the four subscales ranging from 0.76 to 0.86. The results of confirmatory factor analysis indicated that there was a good fit between the hypothesized model and the observed data (e.g., goodness-of-fit index = 0.95, normed fit index = 0.95, comparative fit index = 0.96). For a complete discussion of the development of the atcls and its use, the reader can see Cheung (2009a, 2009b).
The four subscales and items.
| Subscale | Item |
|---|---|
| Liking for chemistry theory lessons | Q1. I like chemistry more than any other school subjects. |
| Q5. Chemistry lessons are interesting. | |
| Q9. Chemistry is one of my favorite subjects. | |
| Liking for chemistry laboratory work | Q2. I like to do chemistry experiments. |
| Q6. When I am working in the chemistry lab, I feel I am doing something important. | |
| Q10. Doing chemistry experiments in school is fun. | |
| Evaluative beliefs about school chemistry | Q3. Chemistry is useful for solving everyday problems. |
| Q7. People must understand chemistry because it affects their lives. | |
| Q11. Chemistry is one of the most important subjects for people to study. | |
| Behavioral tendencies to learn chemistry | Q4. I am willing to spend more time reading chemistry books. |
| Q8. I like trying to solve new problems in chemistry. | |
| Q12. If I had a chance, I would do a project in chemistry. |
From 2009, the secondary schooling in Hong Kong was shortened from seven to six years (i.e., Secondary 1-6). Chemistry is offered as a separate subject to Secondary 4-6 students (approximately 16-18 years of age). Academic year in the secondary schools begins in September every year. I invited two chemistry teachers to evaluate their Secondary 5 students’ attitudes toward chemistry lessons in November 2010. Both Gavin and John (pseudonyms) got a Bachelor of Science degree and majored in chemistry. They also received formal preparation in chemistry teaching at my university and obtained a postgraduate diploma in education. Gavin had 10 years of teaching experience, while John had only seven years. The two teachers administered the 12-item atcls to students during their classes. The survey was conducted anonymously and lasted about five minutes.
Using the spss program, student responses to the 12 atcls items were coded on a scale of 1 (strongly disagree) to 7 (strongly agree) so that higher scores represented more positive attitudes. The mean scores on the four subscales were computed and independent-sample t tests were conducted to determine whether the differences of mean scores of the two chemistry classes are statistically significant.
The two teachers were interviewed by me after they received the survey results. They were asked to comment on the survey results and reflect on their curriculum planning and classroom teaching. Owing to limitation of space, this paper concerns only the responses collected from Gavin.
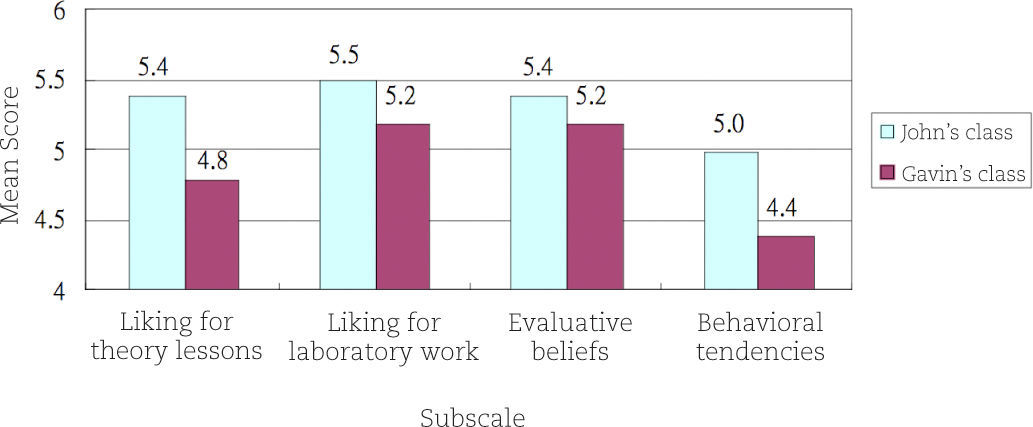
Results and DiscussionThe mean scores for Gavin's class (n = 37) and John's class (n = 31) are shown in Figure 2. On a 1-7 continuum, from ‘strongly disagree’ to ‘strongly agree’ regarding attitude toward chemistry lessons, the mean scores of the two classes are between 4.4 and 5.5, indicating that on average the students of both teachers had a slightly positive attitude toward chemistry lessons. No statistically significant difference was noted between males and females regarding attitude toward chemistry lessons. These findings are largely consistent with those obtained in my previous study (Cheung, 2009b).
Compared with John's class, the attitude toward chemistry lessons was lower among students in Gavin's class. The difference was most noticeable for the mean scores on the fourth subscale and was statistically significant (see Table 2). The students in Gavin's class were less likely to agree that “I am willing to spend more time reading chemistry books”, “I like trying to solve new problems in chemistry” and that “If I had a chance, I would do a project in chemistry.” It can be seen from Table 2 that the difference of the mean scores on the first subscale was also significant. The students in Gavin's class were less likely to agree that “I like chemistry more than any other school subjects”, “Chemistry lessons are interesting” and that “Chemistry is one of my favorite subjects.”
Comparison of two sample means on the four subscales.
| Subscale | John's Class | Gavin's Class | t (df) | Sig. | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| 1. Liking for theory lesson | 5.36 | 0.88 | 4.80 | 1.11 | 2.33 (65.83) | 0.023 |
| 2. Liking for lab work | 5.45 | 0.85 | 5.19 | 0.88 | 1.15 (64.71) | 0.218 |
| 3. Evaluative beliefs | 5.43 | 0.78 | 5.20 | 0.86 | 1.16 (65.58) | 0.249 |
| 4. Behavioral tendencies | 5.04 | 0.76 | 4.42 | 1.10 | 2.73 (63.96) | 0.008 |
The above survey findings were a major concern to Gavin. He was very surprised to learn that the mean score on the Liking for Chemistry Theory Lessons subscale was just 4.8. He expected that the mean score should be higher. During the interview which lasted 45 minutes, he emphasized that the students were free to choose elective subjects. Since they decided to take chemistry, Gavin thought that his students were supposed to possess a positive attitude toward chemistry as a subject in the school curriculum. Two possible reasons were given by him. Firstly, Gavin felt that the teaching schedule was very tight. The Curriculum Development Council (2007) suggested that a total of 270 hours should be allocated to teach the Secondary 4-6 chemistry curriculum (see Table 3). However, Gavin's school could not allocate 270 teaching hours for chemistry. To address the problem of insufficient teaching time, he organized additional lessons during the summer holidays before students moved from Secondary 4 to Secondary 5. The additional lessons lasted 10 days and students needed to study for two hours per day. They mainly covered the sixth topic “Microscopic World II.” Gavin admitted that this topic was difficult for students, particularly the discussions on molecular shapes and the sp2 and sp3 hybridization. Even worse, no chemistry laboratory work or other student-centered activities such as project work and guided inquiry was used as a teaching and learning aid in the summer lessons. Therefore, Gavin reflected that quite naturally, many students did not like the extra chemistry lessons.
The chemistry curriculum for Secondary 4-6 students in Hong Kong.
| Topic | Examples of key concept |
|---|---|
| 1. Planet Earth | Composition of air, electrolysis of sea water, rocks and minerals, thermal decomposition of calcium carbonate. |
| 2. Microscopic world I | Atomic structure, the periodic table, metallic bonding, ionic bond, covalent bond. |
| 3. Metals | Methods of extraction of metals from their ores, word equations, reactivity series, displacement reactions. |
| 4. Acids and bases | Properties of acids and alkalis, ionic equations, indicators, pH, strong and weak acids, molarity, volumetric analysis. |
| 5. Fossil fuels and carbon compounds | Fractional distillation, hydrocarbons, structural formulae, alkanes, alkenes, addition polymerization. |
| 6. Microscopic world II | Bond polarity, van der Waals’ forces, hydrogen bonding, fullerenes, non-octet molecules, shapes of molecules. |
| 7. Redox reactions, chemical cells anelectrolysis | d Primary and secondary cells, half equations and overall cell equations, oxidation number, fuel cells, electrolysis. |
| 8. Chemical reactiand energy | on Standard enthalpy change of reaction, Hess's law, enthalpy level diagrams. |
| 9. Rate of reaction | Instantaneous and average rate, factors affecting reaction rate, molar volume of gases. |
| 10. Chemical equilibrium | Dynamic equilibrium, equilibrium constant, reaction quotient, factors affecting equilibrium. |
| 11. Chemistry of carbon compounds | Homologous series, isomerism, enantiomerism, reactions of various functional groups. |
| 12. Patterns in the chemical world | Periodic variation in bonding, melting point and electrical conductivity, properties of transition metals. |
| 13. Industrial chemistry | Vitamin C, Haber process, rate equation, activation energy, Arrhenius equation, catalysis, green chemistry. |
| 14. Materials chemistry | Naturally occurring polymers, synthetic polymers, alloys, liquid crystals, ceramics, nanomaterials. |
| 15. Analytical chemistry | Qualitative and quantitative analysis, separation and purification methods, instrumental analytical methods. |
| 16. Investigative study in chemistry | Scientific investigation, process skills. |
Secondly, Secondary 5 students had to learn organic chemistry when the academic year began in September 2010 (i.e., topics 5 and 11 in Table 3). Although some laboratory experiments were organized by Gavin, most of them were done as teacher demonstration experiments due to insufficient apparatus. Overall, students were allowed to perform less laboratory work in Secondary 5 than in Secondary 4. This problem was compounded by the fact that the full set of chemistry textbooks was not available from the publisher in July 2010 for him to think about and adjust the teaching sequence. Gavin believed that his teaching sequence was problematic. He guessed that his students may have liked chemistry lessons significantly more if he had not followed the textbook but started with the teaching of electrochemistry in Secondary 5 (i.e., the seventh topic in Table 3).
When asked why the mean score on the Behavioral Tendencies to Learn Chemistry subscale was even lower than that on the Liking for Chemistry Lessons subscale, Gavin pointed out that a lot of his students may have lacked a sense of satisfaction. Hong Kong teachers are under great pressure to prepare students for public examinations because university places are limited. To motivate Secondary 5 students to learn chemistry, Gavin required them to take a 15-min revision test every two weeks as part of course work assessment. He felt that the questions were not unreasonably demanding, but about half of the class always failed the chemistry tests or got fairly low scores. This may have resulted in a lack of sense of satisfaction.
Additionally, learner diversity was serious in Gavin's class. He organized supplementary chemistry lessons after school twice per week (about 1.5 hours each). Attendance was not compulsory. Very often, about 20 students were present, but some relatively weak students could not attend due to the need to participate in extra-curricular activities. Thus, he felt doubtful about the effectiveness of his supplementary lessons.
There are many factors affecting student attitudes toward chemistry lessons in school. These factors include public examinations, grade level and gender (Cheung, 2009b). During the interview, Gavin was eager to explore how to make his chemistry curriculum and teaching more attractive to students to foster a more positive attitude toward chemistry lessons. He felt the need to make chemistry lessons more enjoyable and engaging for his students. He also hoped that the number of students wanting to further learn about chemistry would increase in Secondary 6. The strategies that he planned to implement in school include the following:
- •
Reduce the pace of teaching a bit to allow students to master all the key concepts;
- •
Let students carry out a variety of hands-on activities (e.g., perform laboratory work, make molecular models) when teaching organic chemistry;
- •
Construct both low-order and higher-order questions when designing revision chemistry tests to give students a sense of achievement; and
- •
Modify the teaching sequence so that less abstract concepts are discussed in Secondary 4 and 5 (e.g., teach fossil fuels rather than redox reactions in Secondary 4).
Chemistry educators have investigated various aspects of chemistry teaching and learning, but the attitudinal aspect of chemistry learning in school has been neglected in many countries around the world. My small-scale questionnaire survey dealt with chemistry lessons implemented in two secondary schools in Hong Kong and revealed that the 12-item atcls can facilitate Gavin to evaluate his students’ attitudes toward chemistry lessons. Although his mean scores on the four subscales were above the mid point of the rating scale, student responses to the first and fourth subscales were worrying, particularly in light of the fact that the students themselves had opted for chemistry as a subject in the secondary school curriculum. Gavin felt that the 45-min interview was an excellent opportunity to do self-reflection based on the atcls survey results. My next step is to encourage him to chart student attitudinal change over time. For example, he may collect attitudinal data again toward the end of the Secondary 5 academic year. I also encouraged him to talk with some students to identify their needs and concerns.
Although the 12-item atcls was tailor-made for school chemistry in Hong Kong, researchers and teachers in other countries are most welcome to use or adapt the items. Hopefully, the atcls could serve as an effective tool for teachers around the world to collect information about the chemistry lessons experienced by students.