The development of learning progressions is one approach for creating the types of coherent curriculum frameworks that have been identified as predictors for high-performing scores on international stem assessments. We have developed a learning progression that describes how secondary students may build more sophisticated understanding of the structure, properties, and behavior of matter, and that also outlines the connections and relationships among ideas needed to develop more expert understanding. We used data collected from 82 individual interviews with secondary students and from assessments administered to 4000 Us middle school students to characterize how learners select and apply ideas to explain a range of transformation of matter phenomena. We found that most students relied on a limited set of ideas in their explanations, but that with the proper support, even middle school students were able to appropriately integrate ideas involving the structure of matter, conservation, interactions, and energy to provide mechanistic explanations of transformation phenomena.
El desarrollo de progresiones de aprendizaje es una estrategia para generar el tipo de marcos curriculares coherentes que dan lugar a buenos resultados en las pruebas internacionales sobre conocimientos científicos y tecnológicos. Nosotros hemos desarrollado una progresión de aprendizaje que describe cómo los estudiantes de secundaria pueden construir conocimientos más sofisticados sobre la estructura, propiedades y comportamiento de la materia. esta progresión delinea las relaciones entre ideas que los estudiantes deben desarrollar para adquirir conocimientos más avanzados. en nuestro trabajo utilizamos datos recolectados a través de 82 entrevistas individuales con estudiantes de secundaria y en evaluaciones administradas a 4000 estudiantes estadounidenses, con el fin de caracterizar cómo los estudiantes seleccionan y aplican ideas para explicar fenómenos que involucran transformaciones de la materia. Nuestros resultados indican que la mayoría de los estudiantes utilizaron un conjunto limitado de ideas para construir sus explicaciones pero que, con apoyo adecuado, pueden ser capaces de integrar ideas sobre estructura de la materia, conservación, interacción y energía para construir explicaciones mecanísticas sobre cambios en la materia.
As societies grow increasingly dependent on technology, it becomes more important to have a science literate citizenry. For example, making informed decisions about technological advances and products such as genetically modified plants, stem cell research, and whether to use products incorporating nanoscale structures, requires understanding of core ideas of science. In addition, rapid technological changes related to information and communication have led to a shift from a more local to a global society. This shift requires citizens who are literate in 21st century skills (e.g., critical thinking, problem solving, creativity, flexibility, adaptability), so that they can effectively make informed decisions and solve problems related to societal and global issues (Choi et al., 2011).
Creating a coherent path to support learners in developing understanding of the core ideas of science may help build a science literate citizenry (NRC, 2007). In this paper, we describe the characteristics of such a path using a learning progression (LP) for the understanding of the structure, properties, and behavior of matter as an exemplar. Based on assessments associated with this LP, we discuss the successes and challenges in supporting the development of student understanding in those areas.
Theoretical FrameworkThe value of learning progressionsResearchers from the Third International mathematics and science study (TIMSS) found that one of the major predictors of high achievement in the associated international examinations is the presence of a coherent curriculum framework (Schmidt, Wang, & McKnight, 2005). These investigators define a coherent curriculum framework for a discipline as a set of ideas and skills that becomes relatively more sophisticated over time. In addition, they believe that the framework should illustrate the structure of the discipline by specifying how ideas connect to each other. While there was no single approach for defining a coherent curriculum framework, all high performing countries in the TIMSS examinations used articulated frameworks to guide their science and mathematics curricula. In the Us, which lags behind the high-performing countries, analysis of national and state education frameworks for science and mathematics curricula (i.e., content standards), indicates that these documents generally do not build in complexity, addressing instead the same broad range of topics throughout much of grades 1 through 8. In addition, all topics seem to be treated with equal priority.
To help develop a coherent framework to guide science education, the Us has adopted the idea of learning progressions (LPs), which describe what it means to move towards more sophisticated understanding related to a core idea in a discipline (Smith et al., 2006). LPs do not focus solely on end-product understandings, but also illustrate how ideas build upon one another to create new levels of understanding (NRC, 2007). The new Framework for K–12 Science Education incorporated LPs organized around 13 core ideas to help describe the knowledge and skills learners should develop throughout the primary and secondary grades (NRC, 2012). These LPs guided the development of the Next Generation of Science Standards (Achieve, Inc., 2013) and aim to provide a coherent guide for the organization and alignment of science content, instruction, and assessment.
Progression towards greater expertise described by a LP may occur in different ways. Progress may be somewhat linear in nature. In this view, learning occurs in sequential steps that first require developing an understanding of topic A before building understanding of topic B. Alternatively, progress may be modeled as moving towards greater complexity, where new ideas are added to prior understandings to build new and more sophisticated understandings. As new ideas are introduced, prior knowledge may be reshaped and integrated with the new understandings, or old ideas may be discarded (Bransford, Brown, & Cocking, 1999). This latter type of progression is common in science learning where students’ work to develop more scientifically accurate models. For example, when first developing a particulate model, there is no need to distinguish between atoms and molecules. As students build greater understanding of substances and elements, they need a more sophisticated model for such particles. We used this latter model of learning to guide the development of our LP for the nature of matter.
Developing meaningful understandingAs students develop meaningful understandings, they relate new information to existing knowledge, forming connections that incorporate the new information into an organized, integrated knowledge structure (Ausubel, 1968; Linn, Eylon, & Davis, 2004; Taber, 2001). students’ knowledge structures may not always be well organized, but consist of ideas from prior experiences that are not put together in a systematic, consistent manner (diSessa, 1988). Although learners may possess aspects of the relevant knowledge, fragmented and disorganized structures may not allow them to readily access and use it (Taber, 2000; Sirhan, 2007). Thus, they may have diffIculty applying their knowledge to new situations and to solve novel problems. In contrast, connections and relationships among ideas help create well-defIned integrated knowledge structures. experts generally have well-organized knowledge structures that allow them to easily access and apply ideas (Bransford, Brown & Cocking, 1999; Chi, Feltovich, & Glaser, 1981; shin, Jonassen, & McGee, 2003). Thus, instruction should support students in developing integrated knowledge that allows them to choose and relate ideas appropriately and apply them to new problems. to help meet this instructional goal, a LP should specify not only the knowledge and skills required for more sophisticated understanding, but also the relationships and connections among ideas.
Meaningful learning in scienceOne of the goals of science literacy is for learners to be able to explain and make predictions about real-world phenomena and solve problems by selecting and applying ideas appropriately. explaining many phenomena requires incorporating ideas from several different topics. For example, chemical processes like transformations of matter may require ideas related to the structure and properties of matter, conservation, and energy. However, science instruction and assessment often focus on factual knowledge within individual topics. explanations of transformations of matter often focus primarily on ideas related to the structure of matter, leading to descriptions of initial and final states with little attention to intermediate states and what causes or controls the processes. For example, when explaining what happens to a solid when it melts, a typical response might be a description that focuses primarily on the structural differences between the solid and liquid states–the arrangement and relative space between the particles that make up the substance. In contrast, a mechanistic explanation of what happens when a solid melts would integrate ideas such as transfer and transformation of energy and relate them to the changes in molecular motion and the interactions between molecules that lead to the structural differences. Linking ideas related to the structure of matter to the mechanistic details of how matter behaves and what causes that behavior broadens the understanding of a phenomenon and should help students more readily apply their knowledge to new situations.
Ideas related to energy, interactions, the atomic and kinetic theory of matter, conservation and equilibrium are important not only for explaining most chemical processes, but also multiple phenomena across disciplines. Both the new Framework (NRC, 2012) and the College Board Standards for College Success (2009) articulate certain concepts that are important in this regard. “These concepts help provide students with an organizational framework for connecting knowledge from the various disciplines into a coherent and scientifically based view of the world.” (NRC, 2012, p. 83). For instance, the flow of energy and the way in which matter cycles throughout processes are important in star evolution, rock formation, chemical reactions, and the carbon cycle. The Framework calls them crosscutting concepts and the College Board Standards refers to them as unifying concepts. many of the concepts involve connections and relationships among the ideas important for providing mechanistic explanations of phenomena. For example, the flow of energy and cycles of matter relate the structure of matter, conservation, and energy; change and stability involves ideas relate to interactions of matter, energy, rates, and equilibrium.
The cross-disciplinary nature of these concepts should help learners build more integrated knowledge by helping them see the unifying ideas that explain phenomena at all scales in all disciplines. The ability to make these types of connections becomes even more important with the interdisciplinary nature of current and emergent science. However, students often find it difficult to connect scientific ideas (Renström, Andersson, & Marton, 1990). Thus, they often consider every phenomenon as a unique isolated case. Building understanding of these concepts and their relevance across phenomena requires explicit instructional support both within and across disciplines (NRC, 2012).
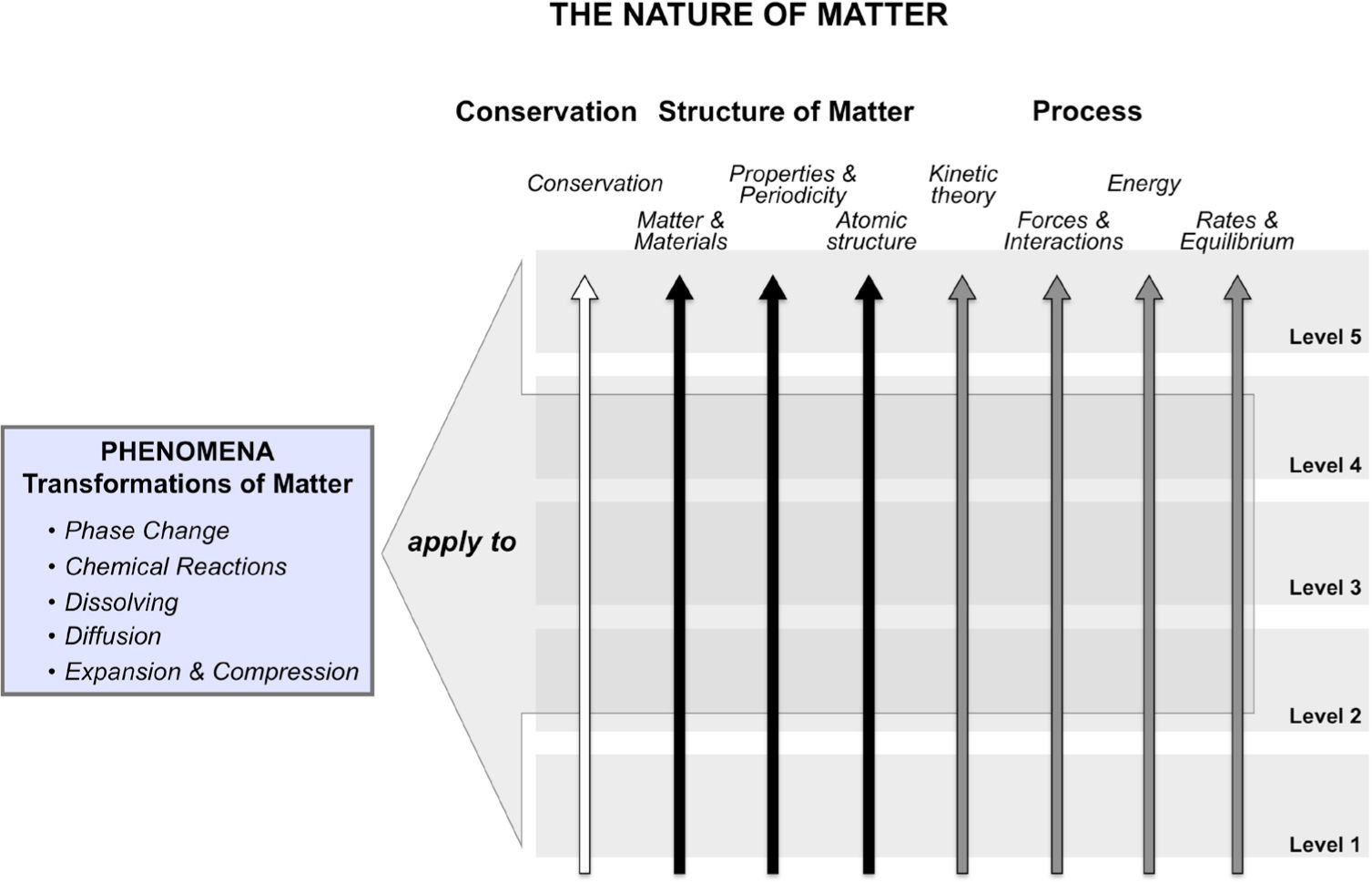
Specifying how ideas connectInitially, we began our work by building a learning progression to describe how students develop more sophisticated understanding of transformations of matter, such as phase changes, chemical reactions, dissolving, and diffusion. However, explanations of all of these phenomena incorporated many of the same ideas — those related to the structure, properties, and behavior of matter. Thus, we shifted our focus to developing a LP for building a more sophisticated understanding of the nature of matter. This LP supports the development of understandings about the relationships between the structure and properties of matter, conservation, energy, interactions, rates, and equilibrium. The proposed LP follows how students incorporate ideas related to these topics into their explanations of transformation phenomena (see Figure 1). We hypothesize that this strategy will provide a guide for instructional support that helps students recognize the similarities across phenomena instead of considering each one in isolation.
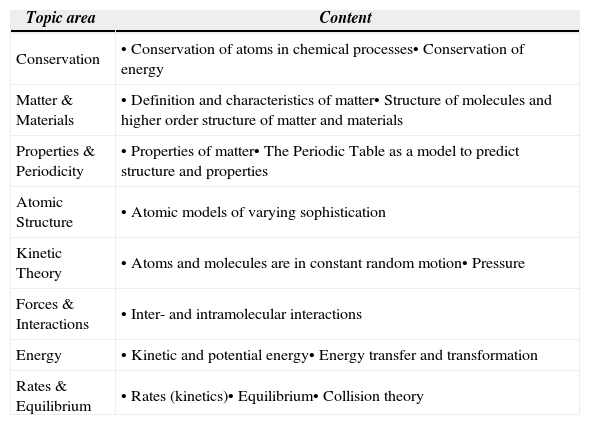
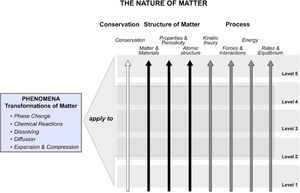
In order to support such learning, each level of our LP includes an integrated set of ideas and skills representing several topics, instead of focusing on single areas of knowledge. This model of a LP provides a path centered on how learners should be able to connect and relate relevant ideas, as opposed to a trajectory driven by factual descriptions of knowledge. Thus, for each level of our LP, we define level appropriate ideas related to the core ideas of a) Conservation, b) structure of matter, and c) Process, which are all important for explaining transformations of matter. As illustrated in Figure 1, the topics of matter and materials, Properties and Periodicity, and Atomic structure are included within the structure of matter umbrella, while the Process (or mechanism) thread includes the topic areas of Kinetic Theory, Interactions, energy and Rates, and equilibrium. table 1 provides a summary of the specific ideas each of these topic areas contains. A description of a portion of the LP has already been published (Stevens, Delgado, & Krajcik, 2010) and the entire LP will be published in detail elsewhere.
Major ideas contained in topics included in the learning progression for the nature of matter.
| Topic area | Content |
|---|---|
| Conservation | • Conservation of atoms in chemical processes• Conservation of energy |
| Matter & Materials | • Definition and characteristics of matter• Structure of molecules and higher order structure of matter and materials |
| Properties & Periodicity | • Properties of matter• The Periodic Table as a model to predict structure and properties |
| Atomic Structure | • Atomic models of varying sophistication |
| Kinetic Theory | • Atoms and molecules are in constant random motion• Pressure |
| Forces & Interactions | • Inter- and intramolecular interactions |
| Energy | • Kinetic and potential energy• Energy transfer and transformation |
| Rates & Equilibrium | • Rates (kinetics)• Equilibrium• Collision theory |
Incorporating ideas from each of the topic areas listed in Figure 1 and specifying how learners should be able to connect and relate these ideas creates a guide for supporting students to build integrated knowledge structures. such structures allow students to appropriately select and apply ideas to new situations. In contrast to purely fact driven knowledge, this approach puts the focus on the connections that learners need to make between relevant ideas and experiences.
While ideas from every one of the topics in table 1 might not be represented on every level, even the lower levels of the LP contain ideas from multiple topic areas. For instance, answering a question such as Why do puddles dry up faster in the summer than in the winter? requires incorporating ideas from several important topic areas even for the lower levels of the LP. At Level 1, learners have a macroscopic model of matter, often relying on real world observations to explain phenomena. A Level 1 response to this question might be: When it’s hotter, water evaporates faster so a puddle will dry up faster in the summer. The response incorporates ideas related to matter and energy, but is quite unsophisticated.
At Level 2, students will develop a basic particulate model of matter and should incorporate those ideas into their responses. A Level 2 response might include ideas such as: Water is made up of molecules that are constantly moving. A few of those molecules may have enough energy to break away from the water and become a gas, so the puddle evaporates. The higher the temperature means that the water molecules move faster, so more of them can escape, making the puddle evaporate faster in the summer than in the winter. At this level, the response also relates ideas about matter and energy, but also incorporates kinetic theory.
Level 3 learners should add other ideas to their responses. For example, they may include ideas about intermolecular interactions and the distribution of energy and motion of particles at a given temperature to help explain how certain molecules have enough energy to break the interactions between them and escape to the gaseous phase. While each level response contains some ideas related from similar topics, the ideas and therefore responses become more complex and sophisticated.
We hypothesize that focusing on sets of ideas to explain phenomena at each level of the LP will support learners in the ability to appropriately select and apply their understanding to explain a range of phenomena. We believe that students at all levels can integrate ideas from multiple topic areas when explaining phenomena. The complexity and scientific accuracy of the ideas that they use in their explanations will change as they move along the hypothetical LP. Thus, as learners progress along the LP, they incorporate new ideas to build more sophisticated models to explain the structure, properties, and behavior of matter. many of these ideas would also be useful in explaining other physical and chemical processes. encouraging integration of ideas related to topics such as energy, interactions, rates, and equilibrium into explanations across phenomena should help students understand the cross-disciplinary nature of these ideas.
The overall goals of our project involve generating and empirically testing a LP for the nature of matter that provides a guide for developing more sophisticated understandings of the structure, properties, and behavior of matter. We developed assessments associated with the LP to create a “ruler” that can be used to place students along the LP and empirically test a portion of the LP. Here we discuss some of our results that illustrate how learners choose and apply ideas associated with the LP, and discuss the successes and challenges related to supporting students in developing integrated knowledge structures.
MethodologyLarge-scale assessmentWe developed items focusing on transformations of matter for each level of the hypothetical LP. the items required students to use and relate ideas from multiple topic areas and apply them in a variety of contexts. We followed the procedure as described by Stevens and collaborators (2010) to develop items with a wide range of complexity and openness (Scalise & Gifford, 2006).
ParticipantsThe developed items were administered to approximately 4000 middle school students varying in race, ethnicity, and socio-economic status (SES) from nine schools in four states across the Us. The schools included seven public, one private, and one charter institution located in urban, suburban and rural schools settings.
InstrumentWe created four test forms each containing 15–20 items for the 6th and 7th grade students (A-version), and four different test forms (B-version) each containing 15–20 items, for the 8th grade students. Only three items differed between the A- and B-version of each form to adjust the overall difficulty of the tests. The test forms were distributed evenly among the students.
Data analysisOpen-ended responses were coded to characterize the ideas students chose to use and apply to the problem (Shin, Stevens, & Krajcik, 2010). For inter-rater reliability, two team members each scored at least 10% of the data and reached a 90% or greater agreement after discussion. Close response items were analyzed using Classical test Theory and Item Response Theory (Wilson, 2005).
Semi-structured interviewsParticipantsInterview data was collected from a total of 82 secondary students from three distinct communities representing a range of race, ethnicity, and SES. Fourteen students were from a public school district serving suburban and rural communities, predominantly Caucasian middle-class communities. Thirty-six students attended a public school district in a diverse, urban community where approximately half of the students were of low SES. The remaining students attended a private grade 6–12 school in an ethnically diverse middle to upper middle class community. The majority of middle school students were in seventh grade. The high school students consisted of two groups, those who were currently or had previously taken chemistry, and those who had not. The students were selected to equally represent gender and the full range of academic abilities as defined by their teachers.
InstrumentA 20-30 minute semi-structured interview was developed to characterize students’ understanding of the nature of matter (Shin, Stevens, & Krajcik, 2010). The topics areas addressed included: the structure and properties of matter; conservation of matter; atomic models; and inter- and intra-molecular interactions. Interviews were conducted with individual students ranging from middle school level to undergraduates.
Data analysisThe interviews were analyzed to identify the ideas students used in their responses. to accommodate all student responses, the coding scheme was based on Min-strell’s (1992) facet approach which defines core ideas within each topic area. The data were coded as described in detail elsewhere (Stevens, Delgado, & Krajcik, 2010).
Classroom ObservationsObservers produced running records using an ethnographic approach that focused on creating detailed descriptions with time tracking of the instructional experience. In order to ensure that team members produced reliable running records that captured the categories included in the observation rubric, three observers achieved reliability by comparing 1) the content of their running records, and 2) comparing and discussing the coding of their running records based on the observation coding scheme.
ParticipantsWe conducted 149 observations of 13 teachers in five schools to characterize students’ instructional experiences for the topics related to our LP.
Data analysisClassroom observations were coded to characterize teacher practice and students’ learning experiences. The coding matrix was developed to align with previously developed teacher survey and curriculum analysis instruments (Kesidou & Roseman, 2002; Minner & DeLisi, 2012). Coding categories and sub-categories (italicized) included (Peek-Borwn et al., 2013):
- •
Lesson set up: purpose, learning goals, contextualization
- •
Learning activity: reading, lecture, laboratory, extended projects
- •
Connections: within lesson, to prior lessons, to real life
- •
Sense-making: connecting ideas, scaffolding observations
- •
Management: related or unrelated to instruction, student disengagement
- •
Inaccurate content: inaccurate representation of science content or processes
- •
Discourse: teacher use of questioning, prompting and providing feedback
- •
Mechanisms: instruction related to the mechanism of a process (vs. descriptive)
Cross-sectional data was collected with secondary students to gain insight into how they select and apply ideas included in the LP for the nature of matter. The data also provided information on the ideas students had difficulty learning and using. We found that at times students were able to appropriately integrate ideas from a range of topic areas into their responses. However, their use and selection of ideas could be inconsistent across contexts. students often prioritized a small subset of ideas, some of which were not always particularly relevant. Here, we will discuss the results from a few items to illustrate how students selected and related ideas to explain aspects of transformations of matter.
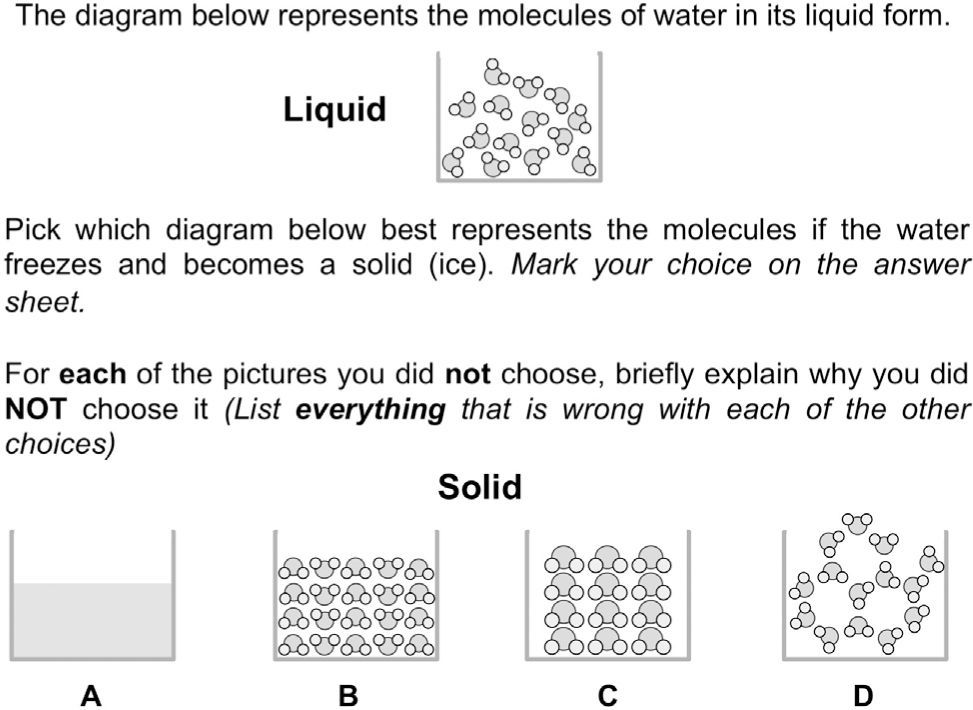
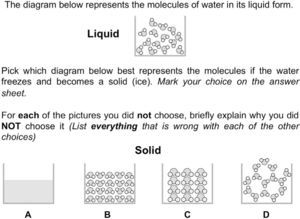
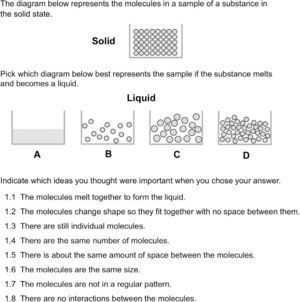
Characterizing students’ responses to the assessment itemsFigure 2 depicts an item that focuses on freezing water. The alternative choices were designed to assess ideas about conservation, characteristics of molecules, the importance of the arrangement of molecules, and the interactions between them through the critique of various models of a solid. While conservation and characteristics of molecules are Level 2 ideas, the importance of the arrangement and intermolecular interactions are Level 4 ideas. Choice A was designed to measure the strength of students’ particulate model and determine whether they hold a Level 1 model of the structure of matter (macroscopic). Approximately 54% of the 899 students indicated that there should still be molecules after the phase change. Another 20% interpreted representation A as a less magnified version of the liquid so we could not assign them definitively to a level on the LP.
When evaluating models B–D, students most commonly focused on the relative amount of space between the molecules in the solid and liquid state. Only 10% of the middle school students chose D as the correct answer. On the one hand, this is not surprising as the hexagonal pattern of water molecules is due to hydrogen bonding, which is not part of instruction until high school chemistry. However, if students prioritized the unchanging number and size of the molecules, they might have found this a more viable choice. Instead, a majority of the students focused on the idea that molecules in a solid should be close together, often believing that model D looked more like a gas or liquid than a solid.
Model B focused on conservation, relative order, and space between molecules in the liquid as compared to the solid state. Approximately 55% of the students chose model B as the correct answer. These students generally prioritized the idea that molecules should be close together in a solid. The significant increase in the number of molecules did not seem important to them as only 6% of the students critiqued the model in terms of ideas related to conservation of matter.
Model C was designed to measure ideas about conservation, characteristics of molecules and relative amount of order between the liquid and solid states. Approximately 30% of the students chose model C as correct. most of those who did not believe this model to be correct fell into two groups; about half of those students believed that the molecules were not close enough to be a solid, while the other half focused on the idea that the molecules should not change their size or shape in a phase change. Only about 8% of the students discussed the need to conserve matter through the phase change.
The amount of space between particles generally decreases only slightly when a material freezes. However, consistent with our observations, it is common for students to exaggerate the extent of the expansion (Harrison & Treagust, 2002). One of the reasons that may lead to students holding such ideas about relative space between particles are inaccurate molecular level representations that commonly depict the liquid particles as being much further apart relative to those of a solid.
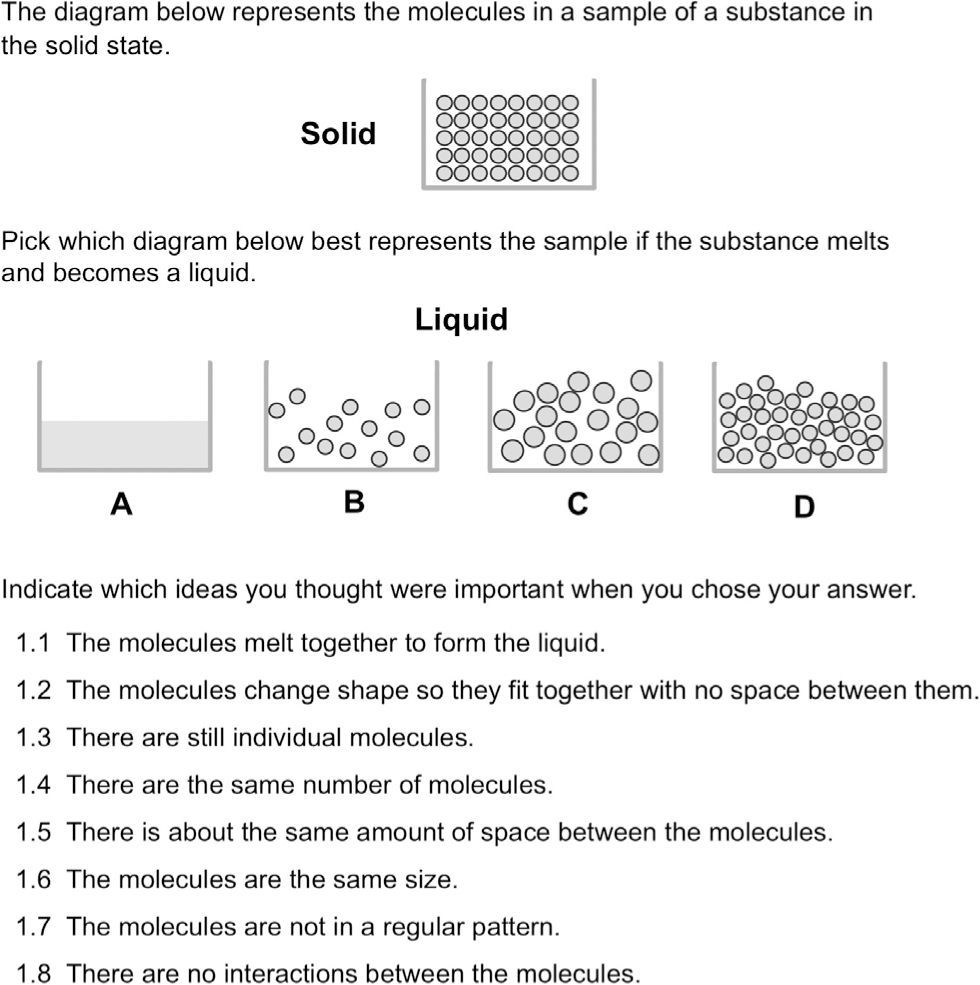
We also developed multiple true/false items that specifically asked students about different aspects of a phenomenon. Figure 3 is an example of an item that measures similar ideas as the open-response item in Figure 2, but in this case focuses on the process of melting instead of freezing. to help decrease the effects of guessing, students were offered a “not sure” option.
Consistent with the open-ended items with a similar focus, most students believed that there should be a significant change in the amount of space between molecules in a liquid versus a solid. even when asked directly, relatively few students believed that molecules can interact with each other only in certain ways. However, students did correctly evaluate some ideas related to interactions between particles. Approximately 50% of students correctly responded that the attraction between molecules is stronger in solids than liquids and that there are still attractions between molecules in the liquid state despite their ability to move freely. These ideas could provide a good foundation for students to build more sophisticated understandings of interactions once they introduce electrons into their models of atoms.
Although very few students considered ideas about conservation in the open-ended context, about 40–60% of students correctly responded that the number of molecules should remain the same through the phase change. While most students readily believed that molecules can slide past each other in the liquid state, a significant proportion (40%) believed that the molecules were not moving in the solid state. Thus, learners did not consistently apply ideas about molecular motion (kinetic theory). Inconsistent use of ideas such as a particulate model or kinetic theory in students’ explanations of different chemical processes is common (e.g., Papageorgiou & Johnson, 2005; García Franco & Taber, 2009). Therefore, learners need coherent instructional support to connect these important ideas to diverse phenomena (Shwartz, et al., 2008).
Students struggled with the relationship between substances and the atoms and molecules that they contain. For example, 43% believed that the molecules themselves change from soft to hard when a liquid freezes; on a different item, 54% believed that one of the reasons ice is harder than water is that the molecules themselves are frozen. These results suggest that students struggle with separating the properties of the bulk substances and those of the atoms and molecules.
Similar trends are observed with other phenomena besides phase change. Figure 4 illustrates an example item related to expansion of a liquid. When asked directly, students were able to respond correctly about ideas related to molecular motion and energy. However, despite their reliance on the change in space between molecules to explain phenomena, a large number of students believed changes to the mass of the liquid in the thermometer occurred when the level rose. similar to their ideas about phase change, students struggled with the relationship between the properties of the bulk substance and individual molecules. Approximately half of the students believed that the molecules themselves got bigger when the liquid expanded. Also similar to the results for phase change, a significant portion of students believed that the molecules that made up a substance were not in motion at lower temperatures.
Even at the beginning of 6th grade, students were able to consider rate-limiting reagents and fundamental ideas about equilibrium. For example, a majority of 6th graders were able to order jars of various sizes by how quickly a candle would be extinguished. In their explanations, approximately one third of the students discussed the relationship between the amount of air or oxygen and how long a candle will continue to burn. In response to another item that asked students what temperature the water would be if a sealed container of ice was left at room temperature overnight, approximately half of the 6th graders predicted that the temperature of the water would be the same as that of the room. two thirds of those students generalized that the water would warm up until it reached the temperature of the surroundings.
Although their understandings may have been based on experiences outside of school, students in early middle school were able to apply those ideas to their explanations of phenomena. Their broad, qualitative descriptions place them in Level 1 of the LP and provide a foundation upon which to build more sophisticated explanations. For example, they seem ready to consider how and why the water temperature cannot rise above room temperature. In this case introducing energy transfer at the macroscopic level would help students begin to develop an understanding of the underlying mechanism. Instructional support is required in order for students to build upon these understandings.
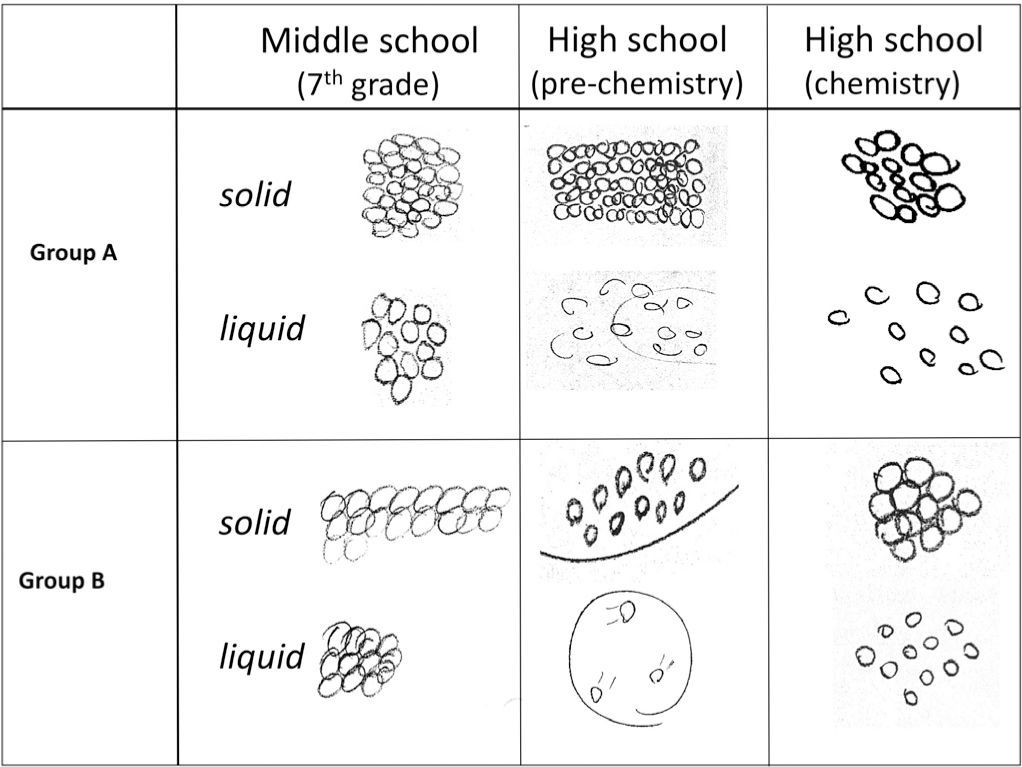
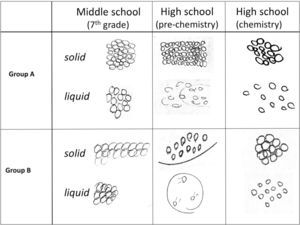
Characterizing student understanding through interviewsThe individual interviews with students provided more complete characterization of students’ models of phase changes. One section of the interview characterized students’ ideas about the process of melting. In an earlier part of the interview, students were asked to explain their model for the structure of a sheet of aluminum, which was followed by a question about what would happen if the metal was heated until it melted. Regardless of their grade level, the models of solid and liquid for students who incorporated particles into their representations (85%) were fairly similar to those illustrated in Figure 5. However, the explanations of their models and what happens during the process of melting differentiated them into two groups. One group tended to focus primarily on structural aspects to explain the process while another group incorporated more process-related ideas into their explanations.
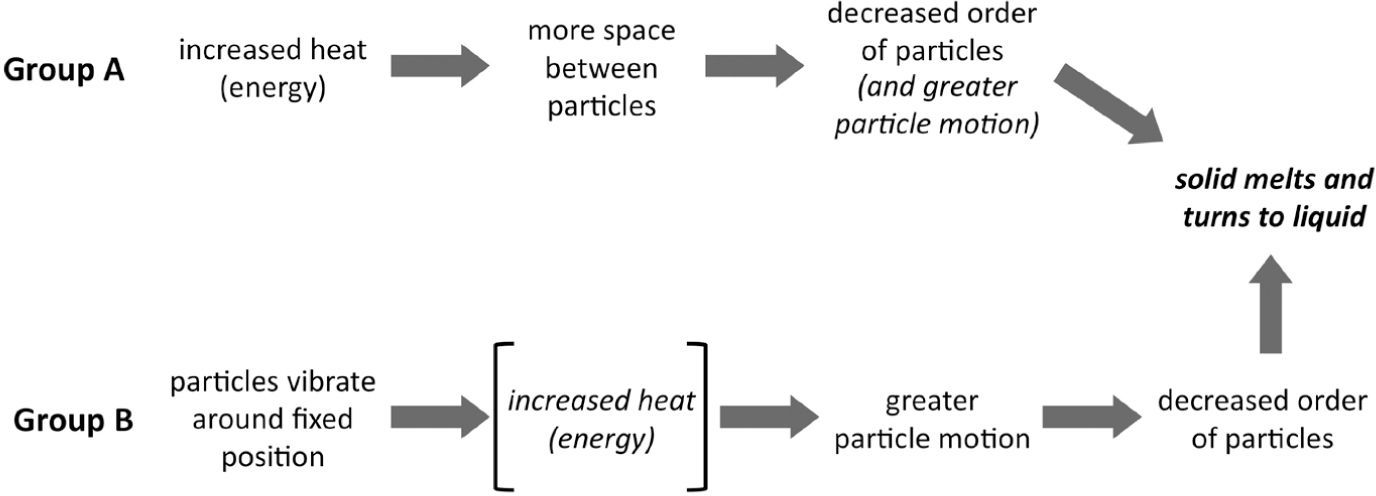
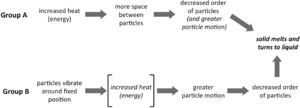
Students in Group A focused more on changes in aspects of the structure between the solid and liquid states and generally neglected ideas about energy and interactions in their models of the process. The relative amount of space between particles was emphasized by 70% of the students. Fifty-five percent of the students also discussed that disorder of the particles increased in the liquid state relative to the solid state and that particle motion also increased. most of the students who incorporated both of these ideas into their response believed that the increase in space between particles drove the mechanism by leading to the disorder of the particles and/or greater motion, and thus the phase change.
In contrast, Group B incorporated more sophisticated range of ideas into their models and generally provided a more accurate mechanistic explanation of the metal melting. Like Group A, essentially all of students 97% incorporated ideas about changes in space between the particles. However, ninety percent of these students also incorporated changes in particle motion into their explanations. The most common explanation involved the particle motion increasing until it becomes so great that the particles can move freely, slide past one another, and decrease the order in the system. many students (42%) explicitly took the additional step of relating the increase in particle motion to the increased heat. Thus, they used the relationship between increasing heat (energy) and particle motion to drive the process. For example, when explaining what happens when a piece of metal melts, a male 7th grade student said: Now the molecules are going to break out of their fixed pattern and start going everywhere. They’d [the molecules] still be tightly packed together but be in more random places…
When asked the follow-up question how do the molecules get out of their fixed pattern? he responded: When they heat up, the molecules start moving faster. And um, when they start moving really fast, they’re — when they’re just attracting and repelling each other. so if they get too fast, they’ll like break apart and go everywhere.
He also indicated that the molecules themselves would be the same size, shape and composition and that the number of molecules would remain unchanged. Thus, this 7th grade student integrated ideas about the structure of matter (molecules, their arrangement and the space between them); kinetic theory (molecules are always in motion); interactions (intermolecular attraction and repulsion); and conservation of matter into his model of phase changes. This contrasts with Group A where the increase in space drove the process by introducing disorder and motion. However, changes in the interactions between the particles during the melting process were rarely discussed by students in either group. Figure 6 summarizes the differences in the models of the two groups. These results indicate that with the proper supports, even middle school students can provide relatively sophisticated explanations of chemical processes and appropriately integrate ideas about particles, their motion, and how energy relates to their behavior into their explanations of phenomena.
We found that students at all grades from Group B were able to integrate the relationship between heat (energy) and particle motion into their models and use that relationship to drive the mechanism of melting. Tus, the more scientifically sophisticated model used by Group B seemed to be differentiated not by grade, but by curriculum (i.e., school or school district). However, in the large-scale assessment, students who experienced the same curriculum but attended different schools did not necessarily integrate ideas with the same level of sophistication. Thus, it appears that teachers’ decisions about the instructional materials play a more significant role in the way students integrate ideas to explain phenomena. Indeed, curriculum analysis for other schools in the large-scale data collection indicated that the instructional materials for most schools also contained models of transformations of matter that included particle motion (kinetic theory) and energy. However, classroom observations suggested that most teachers did not to emphasize these mechanistic ideas, instead focusing primarily on structural ideas. Consistent with the observations, students from these schools generally did not incorporate ideas under the Process umbrella.
Implications and conclusionsWe have found that it is possible for middle school students to provide relatively sophisticated mechanistic explanations of chemical processes. With the proper support, early secondary students can integrate ideas about the structure of matter, the motion of the molecules that make up a substance, and a description of the energy transfer and transformation at the molecular level.
Understanding how and why phenomena occur and how to control them provides students with a foundation for future learning. students generally begin to quantitatively model aspects of transformation processes (e.g., stoichiometry, Le Chatlier, gas laws) in disciplinary courses at the high school level. Without a solid conceptual foundation, students will apply learned mathematical models algorithmically instead of relating them to the appropriate aspects of the phenomena. Conceptually understanding the mechanisms and being able to appropriately select and integrate ideas related to the structure of matter, conservation, energy, interactions, equilibrium, and rates for a range of phenomena within and across disciplines is an important step towards understanding the explanatory power of cross-cutting concepts. In turn, developing these understandings is a key step on the path toward science literacy and becoming citizens who can solve problems and make informed decision about global societal issues of the 21st century.