A well-recognized principle of green chemistry is the use of catalysis to effect chemical transformations. It is difficult, perhaps even impossible to find a current issue of a chemical journal that does not present the use of catalysis, with reports of metathesis-type reactions and of palladium-catalyzed transformations particularly prevalent. Despite the ubiquity of palladium-catalyzed reactions in the practice of synthetic chemistry, however, it is uncommon to find examples of palladium catalysis in the undergraduate laboratory curriculum. In this article, we present our development of such an experiment, beginning from its conception and discussing the progression of modifications and improvements required to convert a reaction as reported in the primary chemical literature to a procedure suitable for the undergraduate organic chemistry laboratory. This presentation, while focused on the development of a laboratory teaching module, serves to illustrate more generally the concepts, principles, and decision-making involved in the practice of green chemistry.
Generations of chemists have regaled their students with stories of hazards they have faced in the laboratory. Explosions, fires, toxic liquids, missing fingers — these have been the trademarks of experimental chemistry from the earliest days of instruction. Sadly, students trained under the assumption that chemistry is intrinsically hazardous — to human health and to the environment — become the practitioners of chemistry in “real life,” and it is small wonder that chemistry in general and the chemical industry in particular are often viewed with distrust and disdain.1 Perhaps beginning with the advancement of the concept of “inherently safer design” by Kletz and Hendershot in the 1970's,2 the chemical industry has increasingly turned its attention to safer alternatives, recognizing that safer chemical processes can translate into higher profits while simultaneously facilitating regulatory compliance and protecting health and the environment.
While the chemical industry, whether motivated by economic, regulatory, or humanitarian concerns, is seemingly embracing the concept of safer chemistry, the academy has perhaps been slower to respond. Faced with concerns about chemical hazards and the spiraling cost of chemical waste disposal, academic teaching laboratories were able to respond simply through reduction in scale, as highlighted by the renaissance of microscale chemistry.3 Only recently has the academy placed an intentional focus on reduction or elimination of the use and generation of hazardous substances — i.e., on green chemistry.4
Green chemistry represents a perfect opportunity for the teaching laboratory, introducing students to the latest breakthroughs in chemical research, preparing them to enter employment in chemical (and chemical-utilizing) industries with awareness of potential hazards and no preconceived notions that hazards are unavoidable, and even saving money through the generation of a reduced and less hazardous waste stream. As a rapidly evolving field, green chemistry presents a unique and powerful opportunity for teachers to highlight the dynamic nature of chemistry. Too long viewed by students as a mature field, with all the important discoveries made long ago, chemistry becomes challenging and thought-provoking when “boundary conditions” regarding safety and environmental impacts are introduced. Particularly effective in imparting this sense of excitement to students is their active and intellectual engagement in the process of conception, design, and development of green chemistry experiments.
As discussed elsewhere,5 the ideal green experiment teaches modern chemical concepts and techniques while simultaneously providing experience with the strategies and principles of green chemistry. This manuscript presents the full process involved in the development of such an experiment, from the initial choice of topic and candidate experiment through the elaboration of a procedure that is reproducibly effective within the confines of a typical organic laboratory session, even in the hands of relatively inexperienced experimentalists. The manuscript concludes with observations regarding opportunities for further refinement, reinforcing for students the dynamic, evolutionary nature of green chemistry.
BackgroundHomogeneous catalysis by complexes of palladium, effecting myriad important and often remarkable transformations, represents one of the most powerful tools in the repertoire of the synthetic organic chemist.6 Indeed, it is difficult to open an issue of an organic chemistry journal without finding several reports of new or improved reactions exploiting palladium catalysis. Despite its ubiquity in practice, however, examples of homogeneous palladium catalysis in the organic teaching laboratory remain uncommon.7 (Heterogeneous catalytic hydrogenation, of course, has historically been well-represented in the organic chemistry curriculum.)
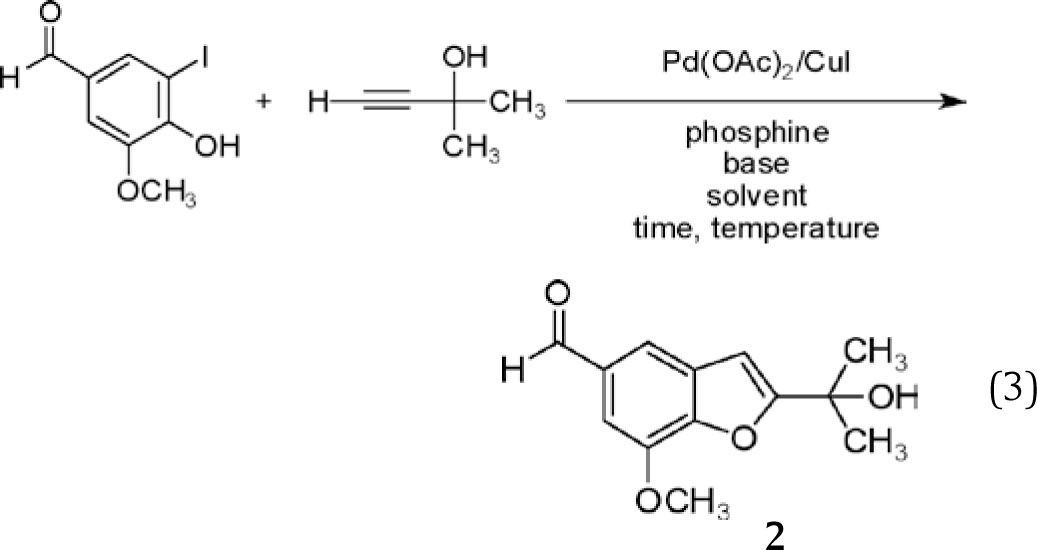
In exploring options for an experiment designed to provide practical experience with the chemistry of alkynes, our attention was drawn to a report by Cacchi, et al., regarding the palladium-catalyzed coupling of terminal alkynes with 2-hydroxyaryl halides,8 a simple example of which is depicted in Equation 1.
In addition to introducing catalysis by complexes of palladium, this coupling reaction highlights a particularly rich range of fundamental organic chemical concepts, including the acidity of terminal alkynes, the addition of alcohols across the alkyne triple bond, and the chemistry of heterocycles (benzo[b]furans, hereafter referred to simply as benzofurans). Thus, it was rather appealing as a laboratory complement to discussions of the chemistry of alkynes in the organic chemistry lecture course. The relationship of the product benzofurans to a variety of plant-derived natural products (Scheme 1) represented an additional attraction, given the intrinsic interest students often find in explorations of the complexity of Nature's chemical designs.
While the procedure reported by Cacchi represents a simple and effective means for the synthesis of a variety of substituted benzofurans, it required substantial modification to fit the constraints of the undergraduate teaching laboratory. The process by which the chemistry reported in this brief communication was adapted to a procedure suitable for the undergraduate teaching laboratory illustrates the concepts, principles, and decision-making of green chemistry, the iterative approach to green experiment design, and the evolutionary nature of green chemistry.
DiscussionThe benzofuran ring system is found in many natural and synthetic products that have potential pharmacological benefits. Given the prevalence of the benzofuran functionality, a number of synthetic approaches to this ring system have been reported. The coupling of 2-hydroxyaryl halides with copper(I) acetylides, reported by Castro,13 is simple and versatile, but requires independent preparation of the acetylides, is typically performed in refluxing pyridine (bp 115°C) or hot N,N-dimethylformamide (120°C), and requires lengthy reaction times (22 h). While representing an excellent example of a chemical reaction that eschews many of the basic tenets of green chemistry — using a stoichiometric heavy metal reagent, performed in solvents presenting moderate (N,N-dimethylformamide) to significant (pyridine) health hazards, and requiring significant energy consumption — this procedure appears far from appropriate for the teaching laboratory.
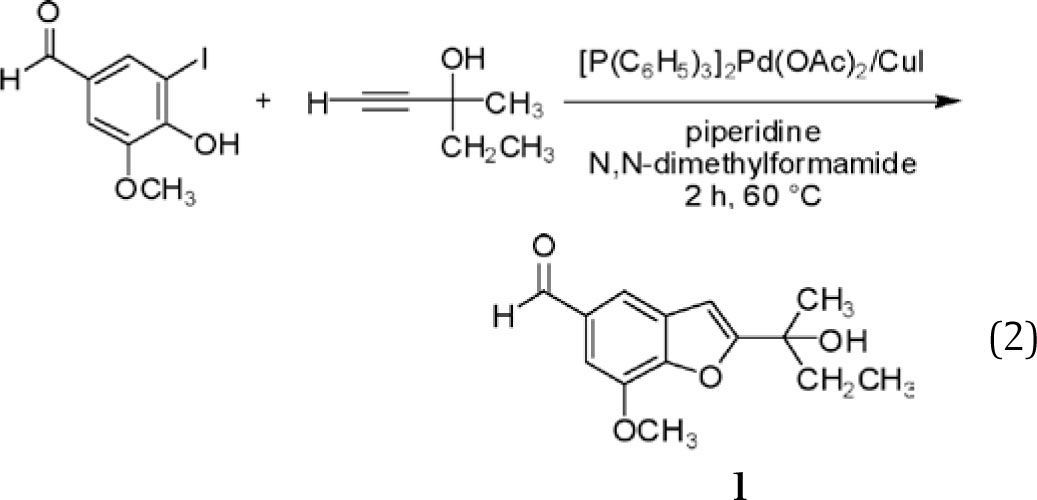
Cacchi, et al.,8 reported an attractive modification of the Castro procedure, in which 2-hydroxyaryl halides were coupled with terminal alkynes (rather than preformed copper acetylides) in the presence of piperidine and catalytic amounts of Pd(II) acetate (2 mol%) and Cu(I) iodide (4 mol%), affording benzofurans in fair to very good yield after 2-10 h at 60°C in N,N-dimethylformamide (equation 2). The benzofuran depicted in equation 2 (compound 1)14 could also be obtained, albeit in reduced yield, with a longer reaction time at 25°C. Following dilution with water and extraction into ethyl acetate, products were purified by flash column chromatography on silica.
We were drawn to benzofuran 1 as a synthetic target for a number of reasons. It was obtained in high yield (87%), exceeded only by a pyridyl derivative obtained from the same alkyne coupling partner in negligibly higher yield. Both precursors are simple and readily available, and one, 5-iodovanillin, is obtainable from the well-known natural product vanillin,15 thereby illustrating an additional green principle — the use of renewable chemical feedstocks. The product bears rather close resemblance to a number of natural products (Scheme 1), providing an interesting connection (as noted above) to the field of natural products chemistry. The 1H NMR spectrum of 1 is complex enough to be interesting, yet readily interpretable. Finally, 1 is a solid (mp 60-62°C), increasing the likelihood that simple precipitation or crystallization could be used in place of chromatography for purification. All this said, however, our initial choice was to focus on a very similar, but not identical target (2) for our experiment development and optimization.
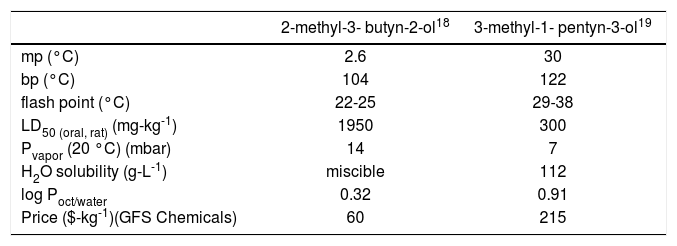
Replacement of the ethyl group in 1 with a methyl group was desirable for several reasons. Benzofuran 2 bears a closer structural relationship to the natural products presented in Scheme 1; indeed, while 2 has not been reported as a natural product, it is so closely related to known natural products that it seems inevitable it will, sooner or later, be identified as also arising from natural sources. With a simpler side chain, the melting point of 2 was anticipated to be higher than that of 1, likely making it easier to crystallize even for students who are still developing their recrystallization skills. (This was confirmed — mp 111-112°C (2) vs. 60-62°C (1).16) Not insignificantly, the alkyne required for the preparation of 2, 2-methyl-3-butyn-2-ol is less expensive than that needed for 1, 3-methyl-1-pentyn-3-ol. Perhaps even more compellingly, examination of the Material Safety Data Sheets (MSDS) for these reactants illustrates that this lower cost is obtained in return for comparable physical properties (Table 1), appreciably lower oral toxicity (to rats), and a lower octanol/water partition coefficient, representing a decreased likelihood for bioaccumulation.17 Put colloquially, “What's not to like?”
In practice, the use of 2-methyl-3-butyn-2-ol in place of 3-methyl-1-pentyn-3-ol proved effective, affording benzofuran 2 in good yield when exposed to the reaction conditions reported for the preparation of 1 (equation 2). This procedure represented an attractive starting point, particularly given its seeming versatility and its utilization of rather low catalyst loading (2 mol%). Several key issues, however, were problematic with regards to its utilization as a green experiment in the teaching laboratory. The requirement for a heterocyclic base — piperidine — called for an exploration of safer alternatives. Use of N,N-dimethylformamide as the solvent, while certainly more attractive than Castro's use of refluxing pyridine, was unappealing due to its safety profile (vide infra) and the difficulty students often face in effecting its complete removal from reaction mixtures. Changes in solvent composition were anticipated to alter the solubility of the palladium catalyst, necessitating consideration of alternatives to the triphenylphosphine complex used by Cacchi. Effecting the reaction at 60°C stood in contrast to the green goal of reducing energy consumption and increased the risk of exposure to N,N-dimethylformamide given its higher vapor pressure at 60°C, while prolonged reaction times at room temperature afforded unacceptably low yields. Finally, use of chromatography for purification of the product was undesirable given the generation of significant quantities of waste, in the form of both the adsorbent and the elution solvent(s).
Thus, we embarked on a process of modification and adaptation of the reaction presented with “generic” reagents and conditions in equation 3, focusing on five key factors — solvent, base, phosphine, reaction conditions, and work-up protocol — in our effort to translate this promising lead into an experiment suitable for the undergraduate organic laboratory curriculum. While the results of these investigations are reported separately below, to the extent possible, these factors are heavily interdependent, making this an excellent opportunity to introduce the concept of “Design of Experiments” if desired.20 This topic, however, is beyond the scope of this manuscript.
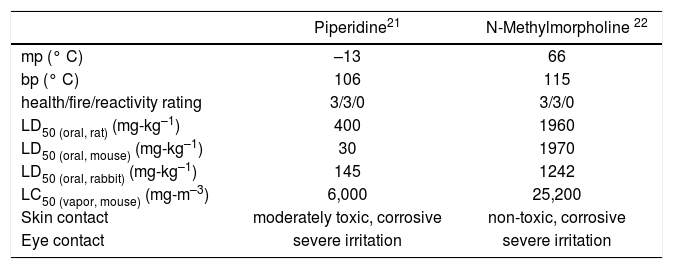
Base. Piperidine presents a sufficient number of health hazards (Table 2) to suggest identification of a replacement whenever possible. N-Methylmorpholine displays similar physical and chemical properties, but appreciably lower oral and inhalation toxicity (Table 2). In addition, although the two compounds bear the same National Fire Protection Association (NFPA) health/fire/reactivity ratings, N-methylmorpholine is appreciably safer in the event of accidental skin contact. Given these issues, we immediately explored replacement of piperidine with N-methylmorpholine and were gratified to find that it was equally effective in mediating the coupling reaction (equation 3) in N,N-dimethylformamide.
Comparison of selected properties of piperidine and N-methylmorpholine.
| Piperidine21 | N-Methylmorpholine 22 | |
|---|---|---|
| mp (° C) | –13 | 66 |
| bp (° C) | 106 | 115 |
| health/fire/reactivity rating | 3/3/0 | 3/3/0 |
| LD50 (oral, rat) (mg-kg–1) | 400 | 1960 |
| LD50 (oral, mouse) (mg-kg–1) | 30 | 1970 |
| LD50 (oral, rabbit) (mg-kg–1) | 145 | 1242 |
| LC50 (vapor, mouse) (mg-m–3) | 6,000 | 25,200 |
| Skin contact | moderately toxic, corrosive | non-toxic, corrosive |
| Eye contact | severe irritation | severe irritation |
Cacchi reported that a more acidic alkyne, ethyl propynoate, did not couple successfully in the presence of piperidine, but found that replacement of piperidine with sodium acetate in this case provided the benzofuran, albeit in modest yield (25%). In our hands, the coupling of 5-iodovanillin with 2-methyl-3-butyn-2-ol in N,N-dimethylformamide using sodium acetate as the base was in fact quite successful, affording benzofuran 2 in 61% yield after chromatography. Posing minimal risk, sodium acetate represents an unarguably greener alternative to piperidine or N-methylmorpholine. The success of this substitution, however, was quite dependent on the nature of the solvent and the reaction conditions, as detailed in the following sections. While the N-methylmorpholine reactions proceeded efficiently when the reactants were briefly mixed, then allowed to stand for 1-7 days, the sodium acetate reactions generally required continuous efficient stirring. Given our laboratory configuration, with multiple students sharing the same space over the period of a week, continuous stirring was not a viable option, leading us to focus primarily on the use of N-methylmorpholine. Ultimately, however, we were able to optimize the sodium acetate procedure so as to obviate the need for continuous stirring, though yields remained somewhat lower than for those reactions in which efficient stirring was maintained for the entire reaction period.
Phosphine. Cacchi reported the use of triphenylphosphine as the supporting ligand for the palladium catalyst, effective given the typically high solubility of (PPh3)2PdX2 complexes in N,N-dimethylformamide. In order to minimize the use of organic solvents, our intention was to explore aqueous reaction media in our experiment design and optimization, suggesting replacement of the hydrophobic phosphine with a hydrophilic analogue, a common strategy in the rather new field of aqueous organometallic chemistry.23 In our first attempt, using a proprietary water-soluble phosphine provided by an industrial collaborator to solubilize palladium(II) acetate in acetonitrile containing ca. 6% water, the coupling reaction proceeded uneventfully, yielding the benzofuran in high yield. Quickly recognizing, however, that a procedure calling for a phosphine of undisclosable structure would be inappropriate, we turned instead to the commercially available water-soluble analogue of triphenylphosphine, sodium triphenylphosphine trisulfonate (TPPTS, 3,3′,3″-phosphinidynetris(benzenesulfonic acid)trisodium salt).24
Further developments using TPPTS are presented in the following discussion of solvent optimization. However, while TPPTS was used in most of the following studies, it is rather expensive. In addition, it is prepared via a decidedly nongreen sulfonation reaction using 100% H2SO4 containing excess SO3. 24 Recognizing that solubility even in heavily aqueous media might not present a problem at the low catalyst loadings used for these coupling experiments, we explored simple use of triphenylphosphine as the ligand for palladium. The results of these investigations, which were only partially successful, are embedded within the following section. In our final procedure, we chose to retain TPPTS as the ligand, largely because it serves as a prototypical example of the use of water-solubilized ligands in the transfer of organometallic chemistry from organic to aqueous media.
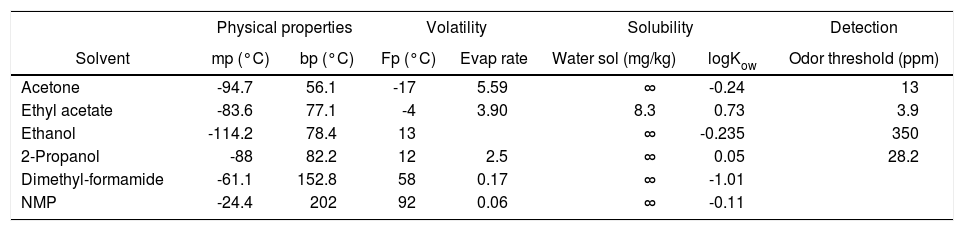
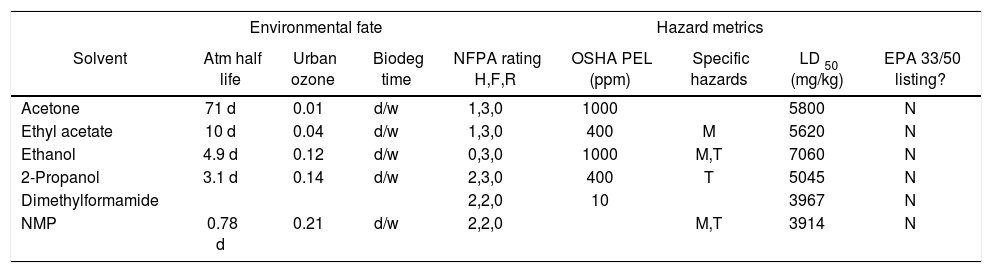
Solvent. Pleased with the initial success of our base replacement studies, we turned our attention to the issue of solvent, seeking to replace the problematic N,N-dimethylformamide with a more benign solvent. N-Methylpyrrolidinone (NMP) has been advocated as a safer polar aprotic solvent, with relatively low volatility and toxicity (Tables 3 and 4), although it has recently been listed as a reproductive hazard in California (2001) and by the European Union (2003).25 The coupling reaction in aqueous NMP (4:1 H2O/NMP), using N-methylmorpholine as the base, gave excellent conversion to the benzofuran. However, while N,N-dimethylformamide was easily removed in workup of the reaction through organic/water partitioning, removal of NMP was more problematic, consistent with the relative octanol/water partition coefficients for the two solvents (Table 3).
Comparison of selected solvent properties.
| Physical properties | Volatility | Solubility | Detection | ||||
|---|---|---|---|---|---|---|---|
| Solvent | mp (°C) | bp (°C) | Fp (°C) | Evap rate | Water sol (mg/kg) | logKow | Odor threshold (ppm) |
| Acetone | -94.7 | 56.1 | -17 | 5.59 | ∞ | -0.24 | 13 |
| Ethyl acetate | -83.6 | 77.1 | -4 | 3.90 | 8.3 | 0.73 | 3.9 |
| Ethanol | -114.2 | 78.4 | 13 | ∞ | -0.235 | 350 | |
| 2-Propanol | -88 | 82.2 | 12 | 2.5 | ∞ | 0.05 | 28.2 |
| Dimethyl-formamide | -61.1 | 152.8 | 58 | 0.17 | ∞ | -1.01 | |
| NMP | -24.4 | 202 | 92 | 0.06 | ∞ | -0.11 | |
Fp = Flash point (°C).
Evap rate = Evaporation rate (relative to butyl acetate).
Water sol = solubility (g/100 mL); ∞ = miscible.
logKow = log (octanol-water partition coefficient).
Comparison of selected solvent properties, continued.
| Environmental fate | Hazard metrics | |||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | Atm half life | Urban ozone | Biodeg time | NFPA rating H,F,R | OSHA PEL (ppm) | Specific hazards | LD 50 (mg/kg) | EPA 33/50 listing? |
| Acetone | 71 d | 0.01 | d/w | 1,3,0 | 1000 | 5800 | N | |
| Ethyl acetate | 10 d | 0.04 | d/w | 1,3,0 | 400 | M | 5620 | N |
| Ethanol | 4.9 d | 0.12 | d/w | 0,3,0 | 1000 | M,T | 7060 | N |
| 2-Propanol | 3.1 d | 0.14 | d/w | 2,3,0 | 400 | T | 5045 | N |
| Dimethylformamide | 2,2,0 | 10 | 3967 | N | ||||
| NMP | 0.78 d | 0.21 | d/w | 2,2,0 | M,T | 3914 | N | |
Atm half life = Atmospheric half-life (h = hours, d = days, y = years).
Urban ozone = Urban ozone formation potential (relative to ethylene).
Biodeg time = Biological degradation time (d = days, w = weeks).
NFPA = National Fire Protection Association ratings for health, flammability, and reactivity (0-4 scale, 0 = safest).
OSHA PEL = Occupational Safety and Health Administration permissible exposure limit (ppm).
Specific hazards: M = mutagenic, T = teratogenic, C = carcinogenic, Corr = corrosive.
LD50 = Lethal dose for oral ingestion (mg/kg).
EPA 33/50 listing = Listing in Environmental Protection Agency 33/50 program (33% reduction by 1992, 50% by 1995).
Attempted replacement of N-methylmorpholine with sodium acetate was unsuccessful in either 4:1 H2O/NMP or in pure NMP, with incomplete conversion even after 6 days of continuous stirring. However, 2 days of stirring with sodium acetate in 1:1 H2O/NMP did afford complete conversion to the benzofuran, even using triphenylphosphine in place of TPPTS, although again isolation of the product from the NMP was problematic.
Both the difficulty in complete removal of NMP and the marginal improvement in greenness relative to the use of N,N-dimethylformamide suggested that we continue our solvent replacement studies. Given our successful transfer of the reaction from N,N-dimethylformamide to aqueous NMP, we felt it was unnecessary to continue to focus on polar aprotic solvents. Anticipating potential issues with solubility in aqueous ethanol, we chose to utilize aqueous 2-propanol, displaying analogous physical and chemical properties with the exception of a larger octanol/water partition coefficient (consistent with the desired higher hydrophobicity). While bearing a higher NFPA health rating, 2-propanol is widely viewed as a very safe compound, representing the main component of “rubbing alcohol.” Using N-methylmorpholine and TPPTS, the coupling was successfully effected in 4:1 H2O/2-propanol, both with continuous stirring for 1-7 days and with initial stirring for 1-2 hours, followed by storage without stirring for 1-7 days.
Replacement of N-methylmorpholine with sodium acetate was effective for the continuously stirred reaction in 4:1 H2O/2-propanol, affording an overall very green procedure. Unfortunately, however, sodium acetate in 4:1 H2O/2-propanol resulted in only low conversion to the benzofuran when stirring was ceased after 2 hours and the reaction mixture was allowed to stand for 7 days. Feeling we were close to a welloptimized procedure, we explored one additional adaptation, decreasing the water content of the solvent to a 1:1 H2O/2-propanol mixture. With this final change, an initial 1 hour of stirring, followed by storage for 1-7 days, reliably provided the benzofuran, even with sodium acetate as the base. While this was meant to be our final modification of the reaction, an inadvertent trial with 1:1 H2O/2-propanone (acetone) instead of 1:1 H2O/2-propanol (making something of a statement about the importance of reading labels carefully!) was equally successful.
Reaction Conditions. Time, temperature, and stirring issues represented the three major variables requiring optimization. For the typical teaching laboratory, time is the most critical issue — in general, an experiment either must be completed within a single laboratory session (generally 3-4 hours) or must be effective even if a full week passes before its completion. As discussed above, this coupling reaction lent itself very naturally to the latter, with the reaction set up in one laboratory period and the product isolated during a subsequent period 1-7 (or more) days later.
The majority of Cacchi's coupling reactions were carried out at 60°C, in many cases providing satisfactory yields of products after only 2 hours, and we confirmed that relatively brief reaction periods at this temperature were similarly effective for the synthesis of benzofuran 2. However, we made the early decision to develop reactions that were effective at ambient room temperature in order to focus attention on the green principle of minimizing energy consumption as well as to deal with practical limitations precluding extended heating in our laboratory setting.
As discussed throughout the preceding section, particularly problematic in our development of this experiment was the issue of stirring. While arguments against continuous stirring could include the avoidance of electrical energy consumption, in our setting it was primarily more pragmatic concerns that dictated our decision to avoid prolonged stirring — with a single laboratory shared by multiple groups of students, maintenance of reaction mixtures on the open, shared laboratory benches was simply not an option. Having successfully developed a protocol that does not require stirring, however, we would not return to the stirred procedures even if our laboratory situation allowed it.
Work-up Protocol. Cacchi routinely purified the benzofuran coupling products by flash column chromatography. While chromatographic techniques are part of the standard repertoire of the synthetic chemist and are frequently introduced in teaching laboratories, chromatography represents a source of considerable waste in the form of the chromatography stationary phase and the eluting solvent(s). Working with crude products obtained from the various modifications discussed above, our exploration of simplified work-up protocols followed a rather circuitous path. Initial efforts to avoid chromatography led to lengthy procedures involving repetitive solvent extractions and treatment with activated carbon, ultimately resulting in appreciable product loss and in the generation of volumes of solid and liquid waste comparable to those from the original chromatographic purification.
In the end, a simple extractive procedure was found to be effective. While 2-propanol is miscible in water, addition of ethyl acetate and an equal volume of water promoted separation into an aqueous and organic layer. After drying and removal of solvent, the benzofuran is obtained as a crystalline solid (sometimes following initial separation as an oil that solidifies upon cooling and scratching the side of the flask). The product (typical mp 108-110°C) can be recrystallized from mixtures of ethyl acetate and hexanes, if desired, leading to formation of nicely crystalline, very pale yellow product (mp 111-112°C). This simple procedure nicely illustrates an additional principle of green chemistry — the minimization of usage of separation agents or other auxiliaries.4
Future DevelopmentsThrough the ages, skeptics have claimed that “there is nothing new to discover.” 26 Through generations of conventional chemistry laboratory courses, students have felt the same way, reproducing time-tested procedures that beg the definition of “experiment.” While the final palladium-catalyzed coupling procedure developed above is significantly greener than the original report upon which it is based, there is room for further improvement, and this should form the basis for ongoing discussions and/or experimentation, further emphasizing for students the stepwise, evolutionary nature of green chemistry and the dynamic nature of chemistry in general. (As we stress to our students, if we wait to make any changes until we have a perfectly green alternative, we may never change. Incremental steps toward greenness, if taken wisely; are better than taking no steps at all.) Several possible directions for discussions and/or experimentation are presented here, in the form of questions and brief suggestions of possible responses to these questions; others will certainly arise as students engage in these discussions.
The product is isolated from an aqueous reaction medium by extraction with an organic solvent, ethyl acetate, from which water is removed by use of a drying agent, representing an additional source of waste. Could the drying agent be omitted? Since ethyl acetate and water form an azeotrope (8.5% water, bp 70.3°C),27 evaporation of the solvent should in theory result in azeotropic removal of water. Taking a step further away from the reported procedure, could an alternative to solvent extraction be developed for isolation of the product from the reaction mixture? The product is at most sparingly soluble in water, while the reactants and reagents are freely water soluble. Would the product crystallize directly from the primarily aqueous reaction mixture that would result from partial evaporation of the reaction mixture?
The atom economy28 of the reaction is only around 50%, due primarily to the use of sodium acetate (mass 82.0) or N-methylmorpholine (mass 101.2) as the base and to the loss of iodide (mass 126.9). Could a simpler, lower molecular weight base be used? Is the base required at all? Would the reaction work with an aryl bromide or chloride instead of an aryl iodide? For that matter, atom economy is only one measure of the efficiency of a reaction. The base and the alkyne are used in considerable excess, so that the actual waste generation is greater than the atom economy would indicate. Could we reduce the quantities of reagents employed to match more closely the stoichiometry of the reaction?
The coupling reaction requires 5-iodovanillin, prepared independently by iodination of vanillin. The iodine is not retained in the product. Is there a way to couple the alkyne to vanillin itself, displacing only a hydrogen atom and eliminating a reaction step, significantly enhancing the atom economy of the reaction and significantly reducing waste generation? Aromatic carbon-hydrogen bond activation has received significant attention in the literature of organometallic chemistry;29 perhaps hints are available there.
ConclusionsThis experiment illustrates a number of green chemical principles. The reaction, which proceeds in high yield and is reasonably atom economical, is effected by catalysis rather than a stoichiometric metal complex. Although the reaction requires the presence of an organic solvent, benign solvents such as 2-propanol or acetone are effective, and their use is minimized by their dilution with water. The reactions are effected at room temperature and without stirring, minimizing the energy input required. Isolation and purification of the product are effected without use of chromatographic supports. This alkyne coupling/intramolecular addition reaction sequence has been reported8 to be successful for a variety of iodobenzene derivatives bearing adjacent OH or NHR (R = H, alkyl) groups, providing a simple and general route to benzofuran and indole derivatives. The contrast of the mild and comparatively innocuous procedures and reagents used to effect the synthesis of these important heterocyclic compounds with the more traditional routes (involving lengthy reactions in refluxing pyridine or dimethylformamide) to these materials is striking.
Each decision point — from the initial selection of a target molecule and synthetic procedure through the final choice of purification procedure — represented an opportunity to apply the principles of green chemistry to the design and execution of the experiment. Throughout this manuscript, we have presented the thought processes and data, in the form of comparison tables, that informed our decision making, thereby illustrating the type and complexity of information that must be considered to make informed green decisions.
Experimental SectionPreparation of 5-iodovanillin.30 Commercial bleach (sodium hypochlorite, 5.25%, 50.0 mL) was added to a suspension of 5.08 g (0.0334 mol) of vanillin and 7.06 g (0.0425 mol) of potassium iodide in 100 mL of ethanol over a period of 20 min at 0°C (ice/water bath), resulting in a reddish-brown mixture. The cooling bath was removed, and the reaction mixture was allowed to warm for 15 minutes, then 50.0 mL of 10% aqueous sodium thiosulfate were added. The mixture was acidified with 6 N HCl, precipitating the 5-iodovanillin, which was recovered by filtration. The crude product (7.65 g, 82.4% yield) was recrystallized from anhydrous ethanol. IR (KBr) 3178 (vb), 3005 (w), 2982 (w), 2946 (w), 2849 (w), 1665 (vs), 1587 (m), 1573 (m), 1492 (m), 1462 (m), 1417 (s), 1354 (m), 1296 (m), 1260 (m), 1247 (m), 1188 (w), 1165 (s), 1145 (m), 1040 (m), 866 (w), 854 (m), 786 (m), 671 (m). 1H NMR (300 MHz, CDCl3): δ 4.01 (s, 3H), 6.70 (s, 1H), 7.41 (d, 1H), 7.85 (d, 1H), 9.80 (s, 1H).
Palladium catalyzed coupling using N-methylmorpholine. 10 mL of 1:1 2-propanol/water was placed in a 20 mL scintillation vial equipped with a magnetic stir bar. The vial was capped with a rubber septum fitted with two needles one for a nitrogen inlet and one for an outlet — and purged — with nitrogen for 10-20 minutes. 5-Iodovanillin (0.287 g, 0.00103 mol), palladium(II) acetate (0.0100 g, 4.45 ×10–5 mol), 3,3',3”-phosphinidynetris(benzenesulfonic acid) trisodium salt (0.0280 g, 4.92×10–5 mol), and copper(I) iodide (0.0102 g, 5.36 ×10–5 mol) were added to the solution. N-Methylmorpholine (0.300 mL, 0.00273 mol) and 2-methyl-3-butyn-2-ol (0.200 mL, 0.00206 mol) were added by syringe, and the reaction mixture was stirred with a magnetic stirrer for 2 h under nitrogen. The septum was replaced with a screw cap, and the mixture was allowed to stand for one week. The reaction mixture was transferred to a separatory funnel and 10 mL of ethyl acetate and 10 mL of water were added. The organic layer was separated and dried over MgSO4. Evaporation of the solvent afforded the crude product (0.276 g) in a reasonable state of purity. Recrystallization may be effected from hexanes/ethyl acetate (ca. 9:1), mp 108.0-110.0°C. Chromatographic purification raised the melting point to 111.0-112.1°C. IR (KBr) 3392 (sb), 3122 (w), 3090 (w), 3011 (w), 2992 (w), 2981 (m), 2956 (w), 2934 (w), 2865 (w), 2794 (w), 2733 (w), 1673 (vs), 1616 (w), 1592 (m), 1476 (m), 1455 (w), 1403 (m), 1380 (m), 1362 (m), 1334 (s), 1282 (m), 1243 (w), 1220 (m), 1178 (m), 1142 (s), 1109 (m), 1089 (m), 993 (m), 967 (m), 927 (w), 860 (m), 848 (m), 822 (w), 751 (w), 721 (m), 673 (m), 617 (wb), 584 (w), 560 (w), 518 (w). 1H NMR (300 MHz, CDCl3): δ 1.74 (s, 6H), 2.25 (s, 1H), 4.09 (s, 3H), 6.73 (s, 1H), 7.38 (d, 1H), 7.70 (d, 1H), 10.02 (s, 1H).
Palladium catalyzed coupling using sodium acetate. The coupling was effected as described above, using sodium acetate (0.212 g, 2.58 ×10–3 mol), 5-iodovanillin (0.273 g, 9.82 ×10–4 mol), palladium(II) acetate (0.0098 g, 4.4 ×10–5 mol), 3,3',3”-phosphinidynetris(benzenesulfonic acid) trisodium salt (0.0270 g, 4.75 ×10–5 mol), copper(I) iodide (0.0100g, 5.25 × 10–5mol), and 2-methyl-3-butyn-2-ol (0.200 mL, 0.00206 mol).