A novel experiment was prepared to help chemistry educators to improve the stereochemistry curriculum of secondary school students and undergraduate students. Based on polarimetry and Vitamin C, chemistry educators are now provided with an experiment that takes into account the delocalization of electrons, and the interactions with plane-polarized light in atomic bonds.
Se preparó un experimento novedoso para apoyar a los educadores de la química a mejorar el currículo de estereoquímica de la educación de bachillerato y licenciatura. Se presenta un experimento basado en polarimetría y vitamina C que toma en consideración de los enlaces químicos y su deslocalización de electrones, así como las interacciones con el plano de la luz polarizada.
Bensaude-Vincent and Simon (2008) proposed a new philosophical perspective on the negative image of chemistry with their Chemistry: The Impure Science (Linthorst, 2010a). For centuries chemists have been studying objects which are not visible. According to Bensaude-Vincent and Simon, this contributed to the negative image of chemistry (Linthorst 2010b; Linthorst, 2012). Tai and Sadler (2007) statistically investigated the learning process of students who were subjected to context-rich chemistry curricula (Linthorst, 2012). In fact, they found no convincing evidence for a better understanding of chemistry and its concepts by students in comparison with traditional curricula. So following Bensaude-Vincent, Simon, Tai and Sadler, we search for instructional demonstrations that might contribute to a better conceptualization of chemical theories and models by students without solely focusing on contexts. This article is an example of such an approach.
Stereochemical concepts are important in the chemical arena because they are employed extensively, e.g. in biochemistry and asymmetric syntheses. Just like in some other European countries, at Dutch secondary schools, students following pre-university education, are subjected to different aspects of stereochemistry, e.g. structural isomerism, cistrans, enantiomers and optical activity (Franken, et al., 2008; Bekkers, et al., 2006; Volhardt and Schore, 1999). Consequently, chemistry educators are concerned with the teaching of stereochemistry. For example, Lipkowitz et al. (2000) developed a synthesis, to be carried out by undergraduate students, which could lead to a better understanding of stereoselectivity. In their synthesis they determined enantiomeric excess with gas chromatography through the use of a chiral stationary phase.
In comparison with Lipkowitz, et al., the work of Cody et al. (2012) was concerned with the way students visualize molecules instead of organic syntheses. They argued that the difficulty of learning stereochemistry is partly due to ‘the limited ability of some students to visualize molecular structures in three dimensions’. Following Bensaude-Vincent and Simon (2008), this should ultimately be explained by the fact that atoms and molecules are not visible. Nevertheless, Cody, et al. (2012) recommend the use of multimedia-based techniques because this might improve 3D thinking.
In one of the most cited stereochemical articles aimed at chemistry educators, Barta and Stille (1994) introduced their “hands on” approach in which students literally have to use their hands to visualize chiral molecules. Lewis (2010) developed an abstract method with his geometrical ‘proto-center concept’. Just like the methods of Barta, Stille and Cody, et al., Lewis proposed a method as a complement to the traditional teaching method of chirality.
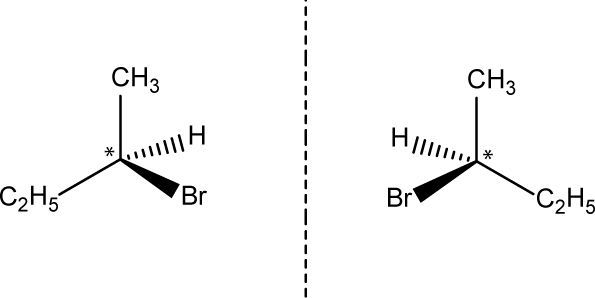
Traditionally, chirality is taught on the basis of asymmetric carbons in a molecule. These carbons are configurationally stable and have four different groups. Considering chemistry didactics, an asymmetric carbon might then be visualized with four different colored balls “covalently” attached to the carbon. Clearly, amongst others Cody, et al. recognize exceptions on this basic assumption, e.g. chiral allenes and biphenyls, but such exceptions do not dominate the learning of chirality (Franken, et al., 2008; Bekkers, et al., 2006; Volhardt and Schore, 1999; Cody, et al., 2012). From secondary school through undergraduate organic chemistry curricula, molecules with one or more asymmetric carbons, dominate in the teaching of chirality. Take for example 2-bromobutane that has one asymmetric carbon (Figure 1).
The mirror images of both 2-bromobutane molecules are not superimposable, and so these molecules are enantiomeric. Both these enantiomers rotate plane-polarized light, but one rotates it to the left and the other one rotates it to the right, which forms the basics of polarimetry (Volhardt and Schore, 1999; Hesse, et al., 1997). In fact, in 2-bromobutane the specific rotation of one enantiomer is + 23,1 deg·cm2·g–1 when the D-line of sodium is employed at 25 °C; the specific rotation of its mirror image is -23,1 deg·cm2·g–1 (Volhardt and Schore, 1999). In comparison with the left-handed 2-bromobutane (C4H9Br) molecule, but under the same conditions (e.g. temperature and wavelength of monochromatic radiation), the specific rotation of the left-handed 2-bromopentane (C5H11Br) differs approximately 10 deg·cm2·g–1 in magnitude (Wamser, 2012). Analogous effects occur in the case of the right-handed ones.
In comparison with 2-bromopentane, the asymmetric carbon (C2) of 2-bromobutane has an ethyl substituent instead of a n-propyl substituent, the other substituents are the same. With respect to the difference in magnitude of the specific rotations: why should a secondary school or undergraduate student accept this difference? One possibility is: by convention, or say a normative idea presented by the teacher. So, considering the colored balls, n-propyl has another color than ethyl. Unfortunately, such an explanation ignores the physical phenomena in which the electric component of plane-polarized light interact with the electrons in molecules and ions. Indeed, drawing from our own experiences, some students might focus on their conceptualization of the atoms that are directly covalently bonded to the asymmetric C2 instead of atoms and bonds along a substituent chain.
For both 2-bromobutane and 2-bromopentane, the atoms bonded directly to the asymmetric center are the same. From this point of view, the colored balls, based on a convention, are not satisfactory for all students. Therefore, we present a demonstration experiment that has the potential to be used by teachers as an adjunct to the other described methods, e.g. the colored balls. This approach is in line with findings of educational psychologists, who advocated the use of experiments and related classroom discussions in science education instead of presenting normative ideas to students (Linn and Eylon, 2006).
For this experiment we used Vitamin C, a compound that appears in several fruits, and which is used in the regeneration of Vitamin E in human bodies (Volhardt and Schore, 1999). Moreover, Vitamin C appears to be suitable for our purpose to focus on the delocalization of electrons in chiral compounds with regards to learning stereochemistry and polarimetry.
Experimental overviewAt room temperature, L-ascorbic acid (> 99% pure) is dissolved in distilled water. This colorless stock-solution (120g/L) is freshly prepared. In several beakers, 20,00mL of this solution is added with a pipette; a volume of 5,00mL of HCl (4M) or NaOH (4M or 6M) is added to each beaker, with distilled water in varying ratios. This results in a series of Vitamin C solutions (96g/L) with different pH values. Subsequently the optical activity of the solutions is determined at 20 °C employing the sodium-D-line (589,3 nm). For this end, a Novex Disc Polarimeter 99.400 Euromex Holland is used. The pH is determined with a Vernier Labquest2 interface. All chemicals are purchased from Boom Meppel BV in The Netherlands.
HazardsConcentrated sodium hydroxide and hydrochloric acid might cause severe skin burns and damage the eyes. Therefore, protecting gloves and goggles should be worn.
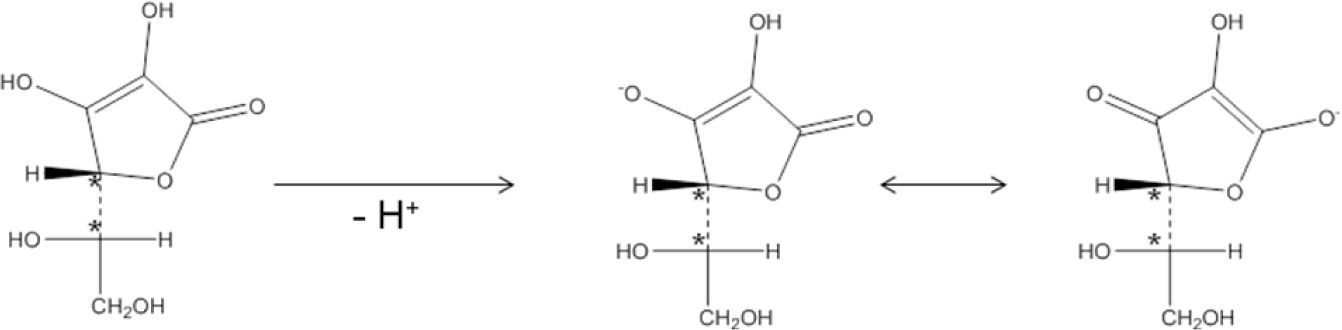
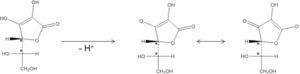
Results and discussionVitamin C, also known as ascorbic acid (H2Z), is electrically neutral and there is no net charge to be stabilized. Following Hückel’s rule, aromaticity is absent in H2Z. Consequently, in H2Z dominates one resonance form in which the oxygens have no formal positive charge (Figure 2). But, with its hydroxyl groups on C2 (pKa2 = 11,7) and C3 (pKa1 = 4,04), H2Z has two enols that are deprotonated with increasing pH (Handbook of Chemistry and Physics, 2012-2013). The OH on C3 is deprotonated first, and gives the ascorbate ion (HZ–). The negative charge of ascorbate is stabilized by resonance, which explains the acidic properties of Vitamin C (Figure 2). Of course, Z2– is also stabilized by resonance.
Vitamin C contains two asymmetric centers on C4 and C5 and is therefore optically active. As shown in Figure 2, after deprotonation the covalent bonds of C4 and C5 remain intact. Nevertheless, these bonds are affected after deprotonation as shown in Figure 3.
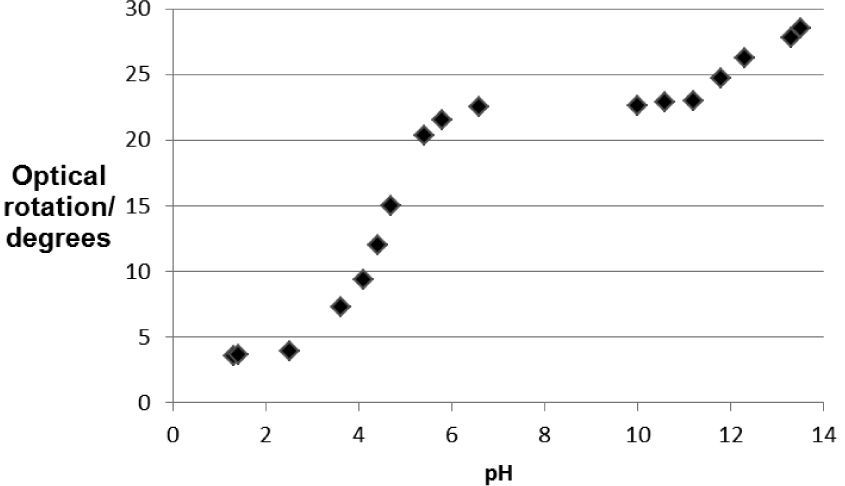
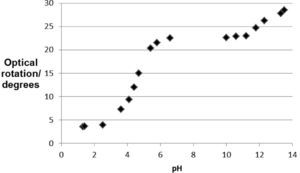
The observed optical rotation is plotted against the pH, which results in a sigmoid shape of the curve in the zone near the mentioned pKa values. At pH < pKa1 - 1 there is a predominance for H2Z in the solution, the optical activity of the solution here is approximately constant. This predominance for H2Z disappears with increasing pH, which starts at approximately pH = 3. At approximately pH = 5, there is a predominance for HZ– instead of H2Z. This decrease in ratio for [H2Z] : [HZ–] is associated with an increased optical rotation of the solution. From pH = 5 to pH = 11, the optical activity of the solution is approximately constant. In this pH zone HZ– is predominant. Starting at approximately pH = 11, HZ– is deprotonated into Z2–, which decreases the ratio for [HZ–] : [Z2–]. This deprotonation gives another accompanied change in the optical activity of the solution because the predominance of HZ– disappears with increasing pH around pKa2. Obviously, H2Z, HZ– and Z2– differ in specific rotation. This shows that there is no straightforward relation between the absolute configuration and the sign of rotation (Volhardt and Schore, 1999).
Contrary to the colored balls, this observation is a springboard for discussion in the classroom that provides a closer look on the matter (Linn and Eylon, 2006). Apparently, the distribution of electrons around the asymmetric centers is affected by the delocalization of electrons around C1, C2 and C3, due to deprotonation of the enols and the resonance of HZ– and Z2–. Contrary to the case of 2-bromobutane and 2-bromopentane, the number of electrons in H2Z, HZ– and Z2– is the same, and thus cannot provide the explanation for the change in optical activity. By now, the teacher has a didactic tool to explain that the mutual distance of electrons in Vitamin C, and its corresponding repulsive forces through the atomic bonds, changes with deprotonation and delocalization. Consequently, the distribution of electrons around the asymmetric centers alters and thereby the interaction of plane-polarized light with these electrons also changes. Indeed, the substituents of the asymmetric carbons in H2Z, HZ– and Z2– are not the same and this difference can be experimentally shown to students.
In sum, with respect to chirality the groups of C4 and C5 in molecular Vitamin C are not the same for ionized Vitamin C. This observation might induce that students are not solely focusing on the atoms directly attached to C4 and C5, but also on atoms and bonds along a substituent chain. For the students it makes sense now to accept the conventional difference (in colored balls) of ethyl and n-propyl, as explained in the case of 2-bromobutane and 2-bromopentane. Without the introduction of complex molecular orbital discussions, this experiment relates macroscopic phenomena and non-visible particles in a way that is disregarded in stereochemistry curricula. This might be changed now with this demonstration, and could eventually be extended with classroom discussions that are concerned with other topics, e.g. structure-activity relationships in stereochemistry and, as a novelty, titration of Vitamin C.
The Royal Holland Society of Sciences and Humanities is kindly thanked for awarding J.A. Linthorst the Pieter Langerhuizen Stipendium in 2010 and The Society for the History of Alchemy and Chemistry is kindly acknowledged for awarding J.A. Linthorst the New Scholars Award 2009. Prof. J.B.F.N. Engberts (University of Groningen, The Netherlands) is kindly acknowledged for his comments on an earlier version of this article.
Author information
J. van der Wal-Veuger is a laboratory technician within the department of chemistry at CSG Dingstede (The Netherlands), which is a secondary school. J.A. Linthorst teaches chemistry at CSG Dingstede and is a PhD student (Maastricht University and Utrecht University, the Netherlands) who investigates the scientific development of green chemistry.